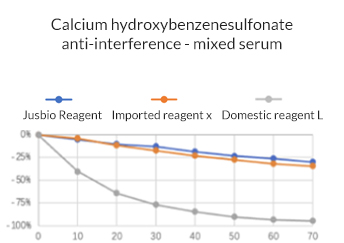
Cholinesterase Assay Kit
Since serum cholinesterase is synthesized by the liver, decreased enzyme activity often reflects liver damage.
Increased: seen in neurological disorders, hyperthyroidism, diabetes mellitus, hypertension, bronchial asthma, type IV hyperlipoproteinemia, renal failure.
Decreased: seen in organophosphorus poisoning, hepatitis, cirrhosis, malnutrition, pernicious anemia, acute infections, myocardial infarction, pulmonary infarction, muscle injury, chronic nephritis, dermatitis, and late pregnancy, as well as ingestion of drugs such as estrogen, cortisol, quinine, morphine, codeine, theobromine, aminophylline, and barbiturates.
Product Features
1. Analytical sensitivity: the change in absorbance should be greater than 0.25 at 5000 U/L.
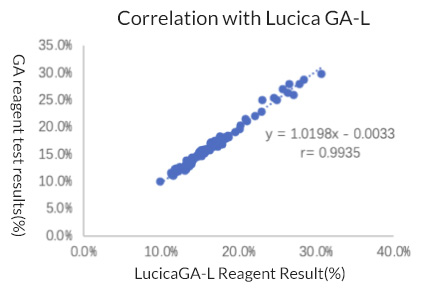
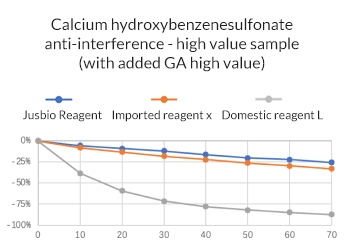
2. Accuracy: Relative deviation within ± 10%
3. Within-run precision: CV ≤ 4.0%.
4. Between run precision: Relative extreme difference R≤5.0%
5. Linear range: (20~15000) U/L, r≥0.990.
6. On-board in use on the analyzer:14 days
7. Expiry date at 2-8℃:12 months