
At the forefront of oncological diagnostics, circulating tumor cells (CTCs) represent a critical component in the battle against cancer. These elusive cells, which break away from primary tumor sites and traverse the bloodstream, hold pivotal information for the early detection, effective treatment monitoring, and accurate prognosis of various cancer types.
Pioneered by Dunwill Medical, the ChimeraX® CTC Detection System introduces a paradigm shift in CTC enrichment methodologies. Utilizing an innovative negative screening approach, this system excels in isolating a comprehensive range of label-free CTC subpopulations. Our technique strategically omits the reliance on surface molecular markers, setting a new standard in precision and efficiency. The process meticulously excludes both white and red blood cells, ensuring an enriched sample of CTCs. Subsequently, advanced immunofluorescence staining techniques are employed to achieve the accurate detection and identification of these critical cells.
This sophisticated approach underscores our commitment to providing cutting-edge solutions in cancer diagnostics, aiding clinicians in making informed decisions and tailoring patient-specific treatment strategies.
Gender: Male; Age: 56
In July 2018, the patient underwent partial hepatectomy, cholecystectomy, common bile duct incision and thrombectomy, as well as T-tube drainage at Zhongshan Hospital Affiliated with Fudan University.
Pathology: HCC grade II G1S4 MVI: M1
Following the surgery, TACE treatment was administered over a month later. CTC was detected 3 months post-surgery, and multiple CTC tests were conducted within one year on after surgery. T-tube chemotherapy was performed in November 2018, December 2018, and February 2019, respectively. In July 2019, an MRI examination revealed left lateral lobe recurrence (17mm).
In July 2019, the patient was readmitted for surgical treatment.
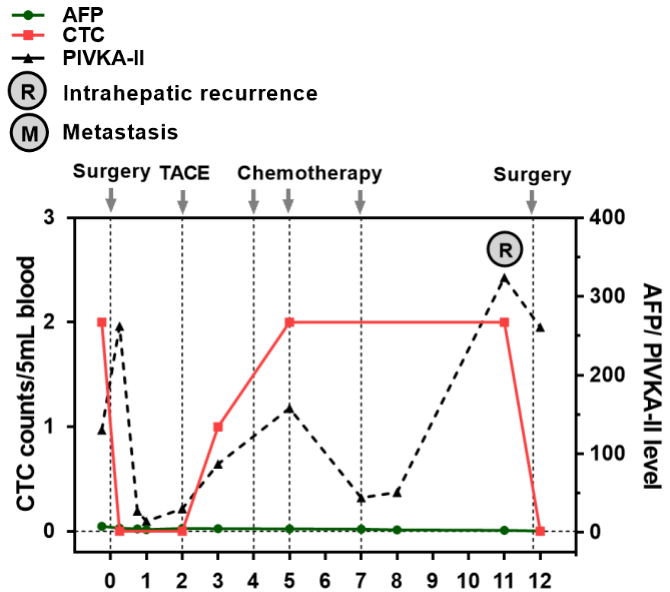
Regular postoperative peripheral blood CTC testing can aid in evaluating treatment response and efficacy, dynamically monitoring disease progression, and indicating recurrence and metastasis at an early stage.



