Significant Role of miRNA7 Test Kit
in Liver Cancer Efficacy Detection and Risk Warning

(PCR Fluorescent Probe Method)
Dynamic Detection of Efficacy in Primary Liver Cancer
Early Warning of Primary Liver Cancer Risk

Human cancers are characterized by several features, including persistent proliferative signaling, evasion of growth inhibition, resistance to cell death, replicative immortality, activation of invasion and metastasis, and induction of angiogenesis. Studies in recent decades have identified that miRNAs are aberrantly expressed in tumors, and dysregulated miRNAs may influence one or more cancer characteristics of tumorigenesis and progression.
Mature miRNAs are very stable in body fluids and have very high specificity across different cancer states, which allows miRNAs to serve as potential non-invasive tumor markers for human cancers.
Patients with hepatocellular carcinoma have been reported to have a specific plasma microRNA expression profile.
Zhongshan Hospital of Fudan University utilized high-throughput microarrays to screen and validate a model consisting of seven microRNAs (miR-21,miR-26a, miR-27a, miR-122, miR-192, miR-223, miR-801) in 934 plasma samples (controls included healthy individuals, chronic hepatitis B patients, and cirrhotic patients), which could accurately differentiate hepatocellular carcinoma from controls.
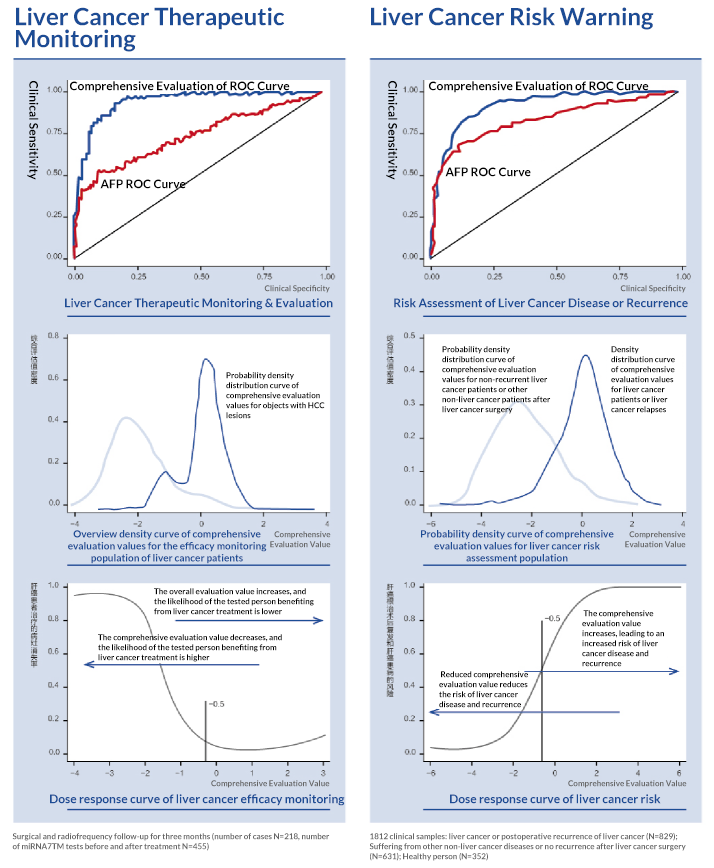
miRNA7 is used for the early diagnosis and dynamic efficacy monitoring of hepatocellular carcinoma (HCC) by detecting the expression of a set of 7 microRNAs in human plasma and analyzing them with logistic regression, obtaining a comprehensive assessment value, comparing it with a threshold value, and determining the negativity and positivity (a positive value is considered to be positive when the comprehensive assessment value is ≥-0.5, and a negative value is considered to be negative when the value is <-0.5).
Safe and Non-invasive
3 ml peripheral whole blood (required to be free of coagulation and hemolysis).
Technical Advantages
Highly efficient target capture by magnetic bead-coupled specific probes and highly specific target detection by optimized design of stem-loop primers and specially modified probes.
Significantly More Effective than AFP
Sensitivity of 76.48%, specificity of 90.03%, national multicenter large-sample clinical trial data (1,812 cases); among the 1,282 AFP-negative samples, the sensitivity reached 77.16% and specificity reached 91.22%.
Early Detection
Detecting cancer signals at the microscopic molecular level enables earlier detection of < 2cm very early stage liver cancer.
Report Time
Equipped with automated pipetting workstation and automated result analysis software, report issued within 6h.
· Throughput of 24-192 samples/run to meet the needs of different volumes of detection.
· Nucleic acid purification and PCR system construction are fully automated, with accurate and stable results.
· Medical device registration (in progress).
· Automatically recognizes and processes output results and generates reports.
· Can be interfaced with systems such as Lims and results are entered automatically.
· Data tracking, summary statistics.

Dynamic monitoring of efficacy in patients with hepatocellular carcinoma

Risk warning for hepatitis B, cirrhosis
and other liver cancer risk groups

Adjuvant determination in a population with
difficulty in distinguishing between benign and malignant tumors

Significant Role of miRNA7 Test Kit
in Liver Cancer Efficacy Detection and Risk Warning
Patient information: A
Sex: male. Age: 64
Case Status
In May 2018, physical examination revealed a large occupancy in the right anterior lobe, which was considered malignant.In June 2018, "segmental hepatectomy" was performed under general anesthesia in the Department of Hepatology. Surgical pathology: hepatocellular carcinoma. Postoperative imaging, AFP and miRNA7 follow-up were performed.Case Discussion
By June 2019, after 6 follow-up visits, no recurrent metastatic foci were found on imaging, and both AFP and miRNA7 indicators were negative, suggesting that the patient's condition was good after surgery, and it was recommended to continue to follow up and monitor the condition; in July 2019, the AFP indicator was negative, and the miRNA7 indicator (1.48) was greater than the comprehensive assessment value (-0.5), which was positive, suggesting that the patient's condition was recurrent after surgery. The patient was diagnosed with recurrence of 9-mm-diameter hepatocellular carcinoma by imaging, and radiofrequency ablation was selected for treatment. After re-treatment, the patient was followed up until December 2019, and the miRNA7 index was negative, and no recurrent metastasis was detected by imaging.Patient information: B
Sex: male. Age: 55
Course of the Disease and Treatment Results
The patient had a history of cirrhosis for more than 20 years, and on July 27, 2020, whole blood samples were collected at regular follow-up, and were tested for miRNA7, AFP, AFP-L3, and PIVKA II, respectively. Among them, miRNA7 (-0.37) was greater than the comprehensive assessment value (-0.5), suggesting the risk of deterioration. on July 28, 2020, MRI examination suggested an abnormal signal in the S7 segment of the liver, and primary liver cancer could not be excluded. After the patient actively improved the relevant examinations, excluding contraindications to surgery, he underwent special hepatic segment + cholecystectomy + extensive intestinal adhesion release under general anesthesia on August 5. The operation was smooth, and the postoperative pathology confirmed the diagnosis of hepatocellular carcinoma, grade III.