Big News! microRNA Testing Has Been Included in the National Medical Service Fee Program!
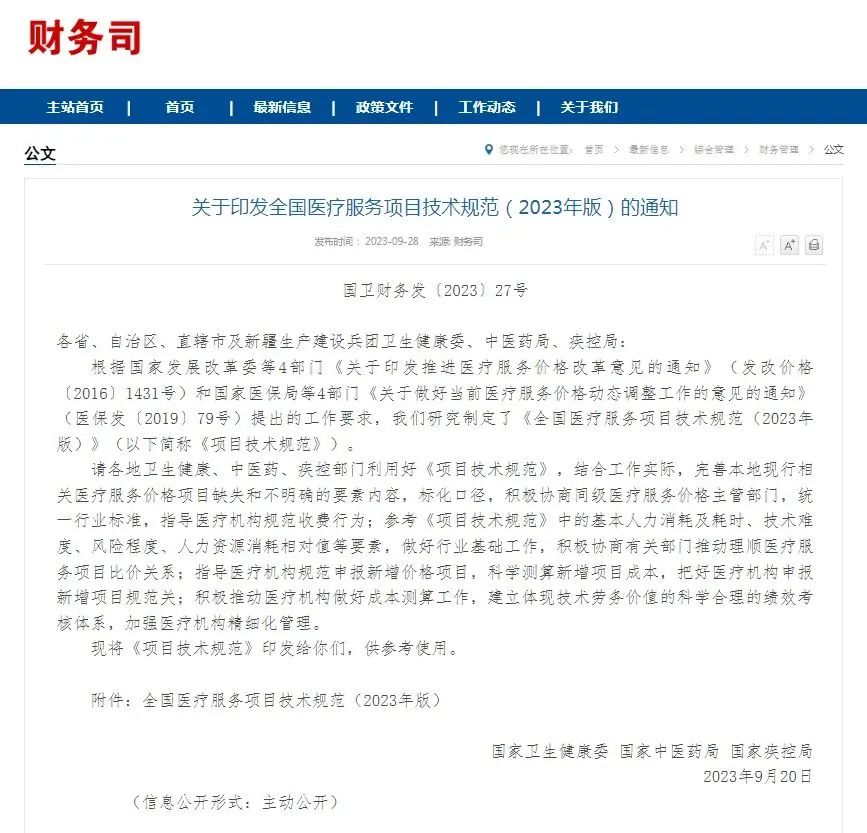
Recently, the National Health Commission, the State Administration of Traditional Chinese Medicine, and the National Bureau of Disease Control and Prevention jointly issued the "National Technical Specifications for Medical Service Projects (2023 Edition)", which includes "microRNA testing" as a national level medical service fee program!
"microRNA" Included in the Medical Service Fee Program
“Project Technical Specification” included microRNA testing services as a medical service fee program at the national level for the first time.
Interpretation
1. miRNA has evolved from an innovative in vitro diagnostic biomarker to an in vitro diagnostic biomarker that can be operated in standard scenarios
As is well known, achieving stability in microRNA detection poses a high technical barrier. Therefore, since 2017, miRNA7™ , as the earliest approved microRNA IVD assay kit, there are still less than 5 microRNA IVD products approved for sales in China. However, this revision of the specification still includes microRNA detection in the new Project Technical Specification, fully demonstrating the accuracy of microRNA testing represented by miRNA7™, as well as its application value in corresponding clinical scenarios, has been highly recognized by the highest regulatory authority in China.
2. miRNA has expanded from existing blood sample testing to various other samples, laying a broader foundation for the clinical application of miRNA
As the earliest approved liver cancer molecular IVD assay kit, the sample applicable type of miRNA7™ is peripheral blood. With the advancement of clinical basic research in recent years, microRNA testing from different clinical samples (such as FFPE tissue, feces, etc.) has been fully demonstrated to have important clinical application value. Therefore, this time the regulation expands microRNA detection from the earliest peripheral blood to various sample types, laying the foundation for the future expansion and application of microRNA in various disease fields.
3. miRNA7™ from Dunwill Medical Effectively promotes miRNA industry standardization through products
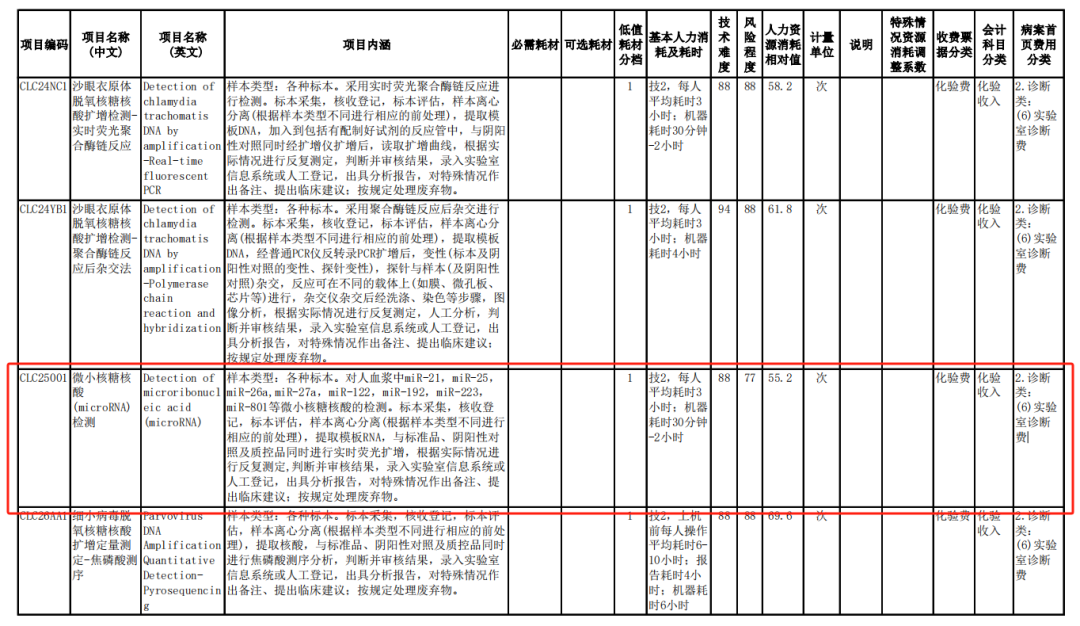
The items defined in this specification for microRNA testing clearly indicate the testing of microRNAs such as miR-21, miR-26a, miR-27a, miR-122, miR-192, miR-223, and miR-801 in human plasma. These microRNA markers cover the 7 microRNA markers of Dunwill Medical's 7 miRNA7TM test kits. Since the product launch of miRNA7™, Dunwill Medical has completed the price declaration, hospital admission, and clinical education for major provinces, cities, and core hospitals nationwide. MiRNA7™, as the first Class III liver cancer molecular testing product approved by the National Medical Products Administration (NMPA) in China, it has won the second prize of the National Science and Technology Progress Award in 2020 and has been included in multiple authoritative guidelines and consensus such as the Diagnosis and Treatment Guidelines for Primary Liver Cancer (2022 Edition). Due to its excellent performance, MiRNA7™ has achieved excellent results in the national multi center consistency evaluation led by the Shanghai Clinical Inspection Center and the results will be published in the journal soon.





