“Sincerity and Adherence to Guidelines”The finalization meeting of the"Diagnosis and Treatment Guidelines for Primary Liver Cancer (2024 Edition)" and the summary meeting of China's liver cancer medical quality control for the year 2023 were grandly held.

“Sincerity and Adherence to Guidelines”The finalization meeting of the "Diagnosis and Treatment Guidelines for Primary Liver Cancer (2024 Edition)" and the summary meeting of China's liver cancer medical quality control for the year 2023 were grandly held in Shanghai recently. The conference brings together top experts and scholars in the field of liver cancer in China, focusing on sharing cutting-edge academic and clinical practice experiences, exploring new knowledge and technologies in the field of liver cancer diagnosis and treatment, interpreting the updated content of the new guidelines, promoting policies and indicators for liver cancer diagnosis and treatment quality control, and summarizing the progress of liver cancer quality control work in 2023.
Fan Jia, an Academician of the CAS and president of Zhongshan Hospital affiliated to Fudan University, made an opening speech for the conference, He said, "In recent years, China's liver cancer prevention, diagnosis, treatment, rehabilitation and other aspects have undergone tremendous changes and made great progress. With the continuous deepening of liver cancer research, improvement of the diagnostic rate, and innovation of treatment methods, more liver cancer patients benefit from it." "Diagnosis and Treatment Guidelines for Primary Liver Cancer (2024 Edition)" is based on the characteristics of Chinese liver cancer and gathered the wisdom of over a hundred authoritative experts from multiple disciplines in the field of Chinese liver cancer, covering high-level evidence-based medicine evidence. It has wide applicability, popularization, operability, and promotability, and has received high response and recognition in various disciplinary fields. In addition, the guidelines also focus on timeliness, constantly updating the progress and latest research methods in the field of liver cancer, and serving as a cross-time guiding document for the diagnosis and treatment of liver cancer.
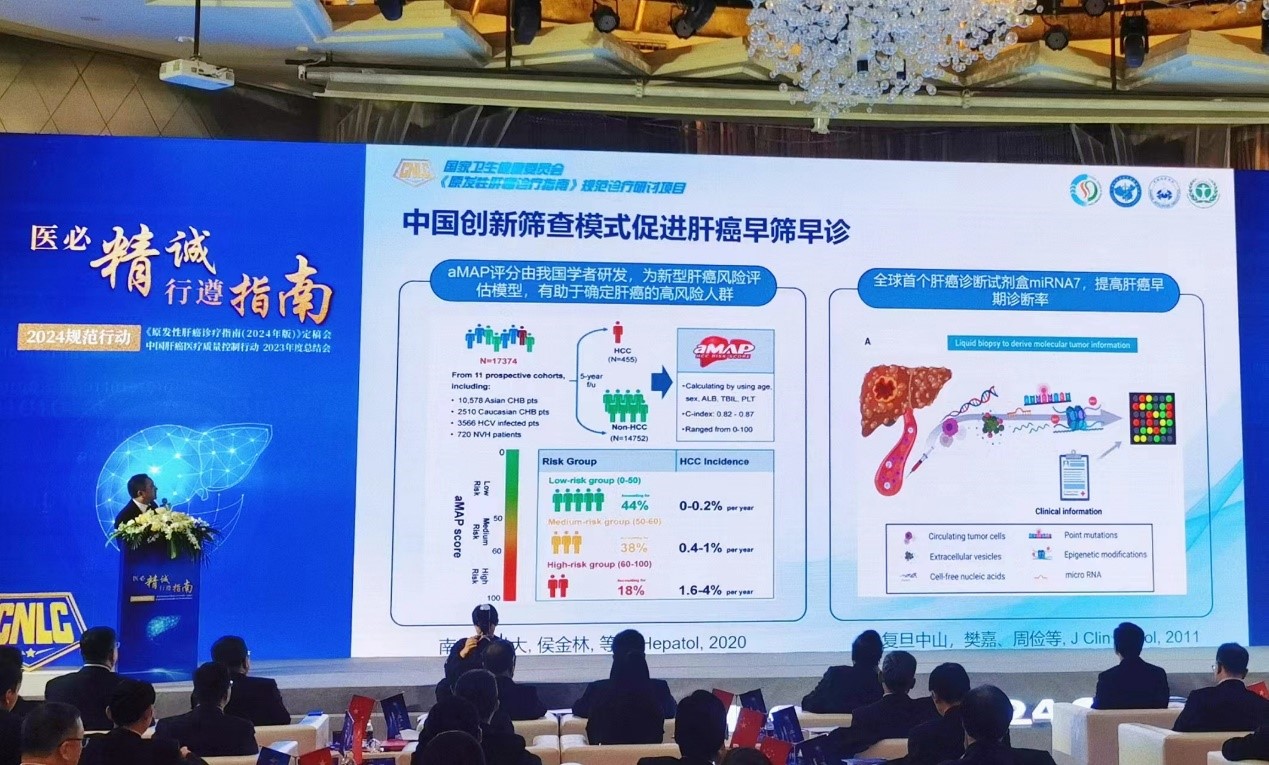
In the subsequent reporting section, Zhou Jian, deputy president of Zhongshan Hospital Affiliated to Fudan University, interpreted the updated key points of the new guidelines. He pointed out that liver cancer is the second leading cause of cancer-related deaths in China. If further control measures including prevention and screening are not taken, it is expected that by 2040, the global incidence and mortality of liver cancer will increase by more than 55% compared to 2020, with nearly half of them occurring in China. In order to meet the needs of diagnosis and treatment, the Chinese liver cancer diagnosis and treatment guidelines/consensus are continuously updated and released. The Chinese Guidelines for the Diagnosis and Treatment of Liver Cancer recommend that high-risk individuals of liver cancer get screening test at least once every 6 months. In addition to traditional screening methods such as ultrasound and serum alpha fetoprotein (AFP), the world's first liver cancer diagnostic kit, miRNA7TM, serves as an innovative screening model for liver cancer in China, helping to improve the early diagnosis rate of liver cancer and promoting early screening and diagnosis of liver cancer.
At the same time, the guidelines have added the content of "regular follow-up for liver cancer patients after surgery" - "liver cancer patients need to have a follow-up visit 1-2 months after surgery, and then closely monitor imaging and changes in oncological markers such as AFP, DCP, and 7 miRNA combinations every 3 months." Through this frequency of follow-up and screening method, the probability of tumors reaching advanced stage will be greatly reduced, and it also provides more powerful evidence-based support for miRNA7TM liver cancer test kits.

The conference commended enterprises that have made outstanding contributions to "Normative Actions" and awarded them the honorary certificate of "Standard Establishment Award". Ms. Sofia Wen, Chairman and Founder of Dunwill Medical, went up the stage as the company representative and received the award from the conference along with representatives from other Fortune 500 companies

At this meeting, professionals and experts in the field of liver cancer diagnosis and treatment from across the country jointly witnessed the finalization of the new guidelines, which has a profound impact on promoting the overall research and clinical practice level of liver cancer in China in the future, as well as promoting the long-term development of standardized diagnosis and treatment of liver cancer. Gathering the power of the guidelines will undoubtedly contribute to the great goal of Healthy China 2030 and create a better tomorrow for the vast number of patients.






