Genome Web Report on The Breakthrough Achievement Published in the top international oncology journal “Cancer Cell” - the First International Mapping of Spatiotemporal and Multi-omics Evolution of Liver Cancer Metastasis

On December 15, 2023, Genome Web reported on the research findings titled "Metastatic Liver Cancer Characterized in Multiomic Study" published in the top international oncology journal Cancer Cell, in collaboration with the team of Academician Fan Jia from Zhongshan Hospital Affiliated to Fudan University, the team of Zhang Liye from Shanghai University of Science and Technology, and Shanghai Dunwill Medical.
Research Content
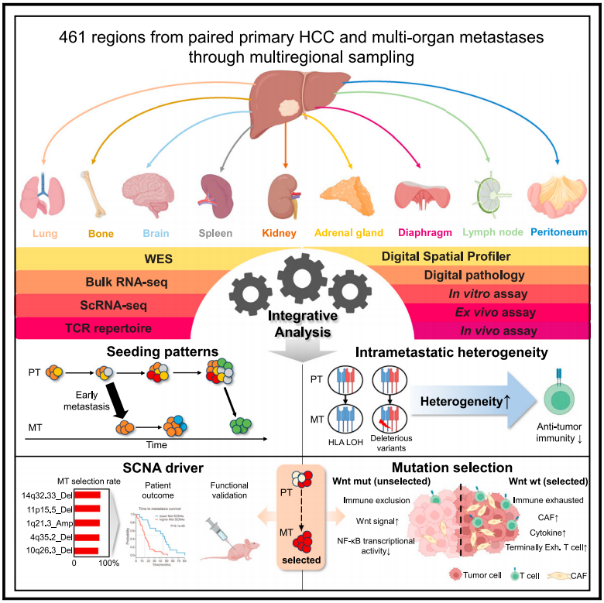
This study screened 182 hepatocellular carcinoma patients from over 20000 liver cancer surgery patients in the liver cancer sample banks of Zhongshan Hospital Affiliated to Fudan University and Tianjin Cancer Hospital. Retrospective multi regional sampling was conducted on surgical resection samples of primary lesions, metastatic lesions, and intrahepatic recurrent lesions. A total of 461 paraffin embedded tissues were collected for joint sequencing analysis including genomics, transcriptome, single-cell transcriptome analysis, multi-omics spatial transcriptome and digital pathology.
This study integrates spatiotemporal and multi omics techniques to draw an evolutionary map of liver cancer metastasis process, revealing for the first time the molecular time at which liver cancer initiates metastasis, the cloning mode of metastasis colonization, the intertumoral heterogeneity of metastatic tumors and their correlation with the microenvironment, the key events driving metastasis, and the mechanism of clone selection during the metastasis process. This research achievement provides a deeper theoretical basis for the precise diagnosis and treatment of liver cancer metastasis.
Genome Web Special Report
Metastatic Liver Cancer Characterized in Multiomic Study
Using a multi-omics approach, a team from China has teased out molecular features found within and across metastatic hepatocellular carcinoma (HCC) tumors over time, uncovering diverse tumor clones showing early metastatic spread.
"HCC metastasis is traditionally viewed as the end-product of disease," Yunfan Sun, a liver surgery researcher with Fudan University's Liver Cancer Institute, explained in an email. "However, our data show a high degree of genetic divergence between primary HCCs and metastases, reflecting that metastatic precursors emerge early in evolutionary history."
As they reported in Cancer Cell on Thursday, co-first author Sun and his colleagues relied on exome sequencing and RNA sequencing to profile 461 formalin-fixed paraffin-embedded tumor regions from 182 individuals with metastatic HCC (mHCC) tumors of the lung, bone, brain, kidney, adrenal gland, lymph node, spleen, diaphragm, or peritoneum.
Based on genomic and transcriptomic profiles, which were analyzed in combination with digital spatial profiling (DSP), histopathology, T-cell receptor repertoire, and single-cell RNA-seq data on the metastatic tumors, the team characterized the genetic heterogeneity found in mHCC tumors.
"Despite progress in surveillance and treatment strategies that have improved overall survival rates, metastasis is still the major cause of patient mortality and remains largely incurable in HCC," Sun said, noting that "better understanding of metastasis is thus desperately needed to improve the prognosis of late-stage HCC patients."
In the process, the researchers flagged suspected driver mutations in the metastatic tumors, including somatic copy number alterations, while also highlighting primary tumor alterations that tend to be lacking in the HCC metastases, such as alterations affecting the Wnt signaling pathway.
"Consistent with previous studies in other cancer types, we do not discover recurrent metastasis-specific mutations," Sun explained. "However, increased global genome instability is evident in metastatic HCCs, suggesting somatic copy number events might play a critical role in enabling metastatic potential."
Using data from a subset of mHCC patients with multi-regional metastatic tumor samples, meanwhile, the investigators distinguished between metastatic seeding events marked by individual tumor subclones or seeding by multiple subclones and then linked polyclonal seeding to poorer patient outcomes.
The genomic divergence found between primary and metastatic tumors also pointed to early metastatic seeding at sites outside of the liver, meanwhile, hinting that there may be a previously unappreciated opportunity to stamp out micro-metastases in individuals being treated surgically with curative intent for localized HCC who might have occult cancer cell spread beyond the liver, Sun said.
He added that "neoadjuvant therapy in patients with resectable HCC might slow tumor progression and prevent further cancer cell dissemination," for example, while "postoperative adjuvant therapy might help eradicate minimal residual disease."
Beyond a new appreciation for the tumor alterations present in the extrahepatic HCC metastases, the investigators went on to explore the relationships between these tumor features, the immune system, and tumor microenvironment.
Although the mHCC tumors were often marked by high intratumor heterogeneity (ITH) that would be expected to produce neoantigen targets for the immune system, for example, their results revealed reduced T cell immune responses to high ITC, high neoantigen tumors — an effect that they attributed to altered antigen presentation capabilities.
"We found evidence that tumor cells from neoantigen ITH-high metastases acquired genetic aberrations that disrupted antigen presentation after their metastatic seeding, which might lead to reduced immunogenicity," Sun explained.
Because past studies have described durable immune checkpoint blockade immunotherapy responses in advanced non-small cell lung cancer cases marked by low neoantigen ITH, Sun noted that the new findings may also offer neoantigen clues for predicting mHCC responses to checkpoint immunotherapy.
From these and other findings, Sun further suggested that "perioperative systemic therapies such as immune checkpoint blockade (ICB) could offer a survival benefit for resectable HCCs, which is of tremendous clinical significance in light of several phase II studies evaluating the role of perioperative immunotherapy."
On the other hand, Wnt-wild type mHCC tumors tended to correspond with a "reactive" or inflammatory tumor microenvironment containing cancer-associated fibroblasts, the authors explained, noting that "reversing the fibro-inflammatory ecosystem might be an effective therapeutic strategy for preventing the onset of metastatic spreading."
Associate Researcher Sun Yunfan stated: “At present, the treatment effect of liver cancer metastasis in clinical practice is not ideal, and there is no solid foundation and translational research, making it difficult to promote the development of new clinical technologies, resulting in a lack of effective tools for predicting liver cancer metastasis and new drugs for preventing or treating liver cancer metastasis. Based on this fact, under the leadership of Academician Fan, we began this study in 2018. Through this large queue integrating multi omics research, we hope to answer a series of core scientific questions about the progression of liver cancer metastasis, such as the molecular time at which liver cancer initiates metastasis, the cloning mode of metastasis colonization, the intratumoral heterogeneity of metastatic tumors and their correlation with the microenvironment, the key evolutionary events driving metastasis, and the mechanisms of clone selection during the metastasis process. In the future, we will focus on the molecular markers closely related to driving liver cancer metastasis discovered in this study, and develop clinical molecular detection kits for early warning and monitoring liver cancer metastasis through clinical translational research; At the same time, on the basis of our existing multi omics, we will further enrich the dimensions of omics, such as adding epigenetics and proteomics, as well as spatial transcriptomics, hoping to discover more molecular targets that can be transformed and applied in clinical practice as metastasis prediction and treatment.”
Academician Fan Jia from Zhongshan Hospital affiliated to Fudan University, Researcher Zhang Liye from Shanghai University of Science and Technology, Associate Researcher Sun Yunfan from Zhongshan Hospital affiliated to Fudan University, and Dr. Peng Haixiang from Shanghai Dunwill Medical are the co-corresponding authors of this study. Associate Researcher Sun Yunfan, Dr. Wu Bin, Dr. Zhang Zefan, Dr. Wang Zejian, Dr. Zhou Kaiqian, Dr. Song Minfang, Professor Ji Yuan, and Professor Zang Fenglin are the co-first authors of this study. This project has been supported by projects such as the National Natural Science Foundation of China and the Shanghai Innovation Cluster. Dunwill Medical and Shanghai University of Science and Technology Supercomputing Center have provided strong support for multi omics data production and bioinformatics analysis.





