“The Multicenter Study on the Comparison of FGFR2 Gene Fusion/Rearrangement Detection Methods in Intrahepatic Cholangiocarcinoma” Project Has Been Officially Launched
Molecular diagnosis has become one of the important cutting-edge fields in contemporary medical development. In recent years, with the emergence of new molecular markers and the continuous development of various innovative molecular diagnostic technologies, the demand for precision medicine continues to increase. It is very important to conduct in-depth research and translate research results into clinical applications. To further standardize the quality management level of molecular diagnostic technology, actively promote the rapid development of molecular diagnostic field in China, and provide more reliable test results for clinical practice, the conference “Progress and Application of New Molecular Diagnostic Technologies" hosted by the Molecular Pathology Center of Zhongshan Hospital affiliated with Fudan University was recently held in Shanghai.
On the morning of December 10th, the consensus seminar & multi center research launch meeting were grandly held. At the beginning of the meeting, Professor Zhou Jian, Vice President of Zhongshan Hospital affiliated to Fudan University, delivered an opening speech. Mr. Zhou said, "In the past five years, the research and development of innovative drugs and the emergence of innovative technologies have greatly promoted the level of diagnosis and treatment of liver cancer in China, while improving the 5-year survival rate of liver cancer patients. In primary liver cancer, the incidence of intrahepatic cholangiocarcinoma is second only to hepatocellular carcinoma, but it lacks early and effective diagnostic methods. Most patients are diagnosed in the late stage, and their quality of life cannot be guaranteed. The survival cycle after diagnosis is very short. Therefore, it is urgent to launch the" Pathological Expert Consensus for Accurate Detection of Intrahepatic cholangiocarcinoma" to further improve the early and effective diagnostic techniques and methods for intrahepatic cholangiocarcinoma." FGFR2 fusion/rearrangement, as one of the most extensively studied targets in clinical studies of intrahepatic cholangiocarcinoma, still lacks clear molecular detection recommendations based on clinical evidence. Therefore, the significance of this conference on "multicenter research on methodological comparison of FGFR2 fusion/rearrangement" is extraordinary, which can provide more sufficient evidence and support for clinical applications.
In the subsequent thematic presentation segment, Dr. Zhang Xin from the Pathology Department of Zhongshan Hospital affiliated to Fudan University presented an exciting report titled "The Multicenter Study on the Comparison of FGFR2 Gene Fusion/Rearrangement Detection Methods in Intrahepatic Cholangiocarcinoma", Dr. Zhang introduced, "Currently, there are many methodologies for FGFR2 fusion/rearrangement detection, but there is a lack of large sample multicenter studies for methodological comparison. The purpose of this multicenter study is to compare the detection of FGFR2 gene fusion/rearrangement using detection methods such as FISH, DNA-NGS, and RNA-NGS. We hope to conduct a multicenter study on the methodological comparison of FGFR2 gene fusion/rearrangement detection in intrahepatic cholangiocarcinoma through this multicenter project.", "Head-to-head comparison of FGFR2 fusion/rearrangement detection methods to improve clinical evidence for precise detection of intrahepatic cholangiocarcinoma."
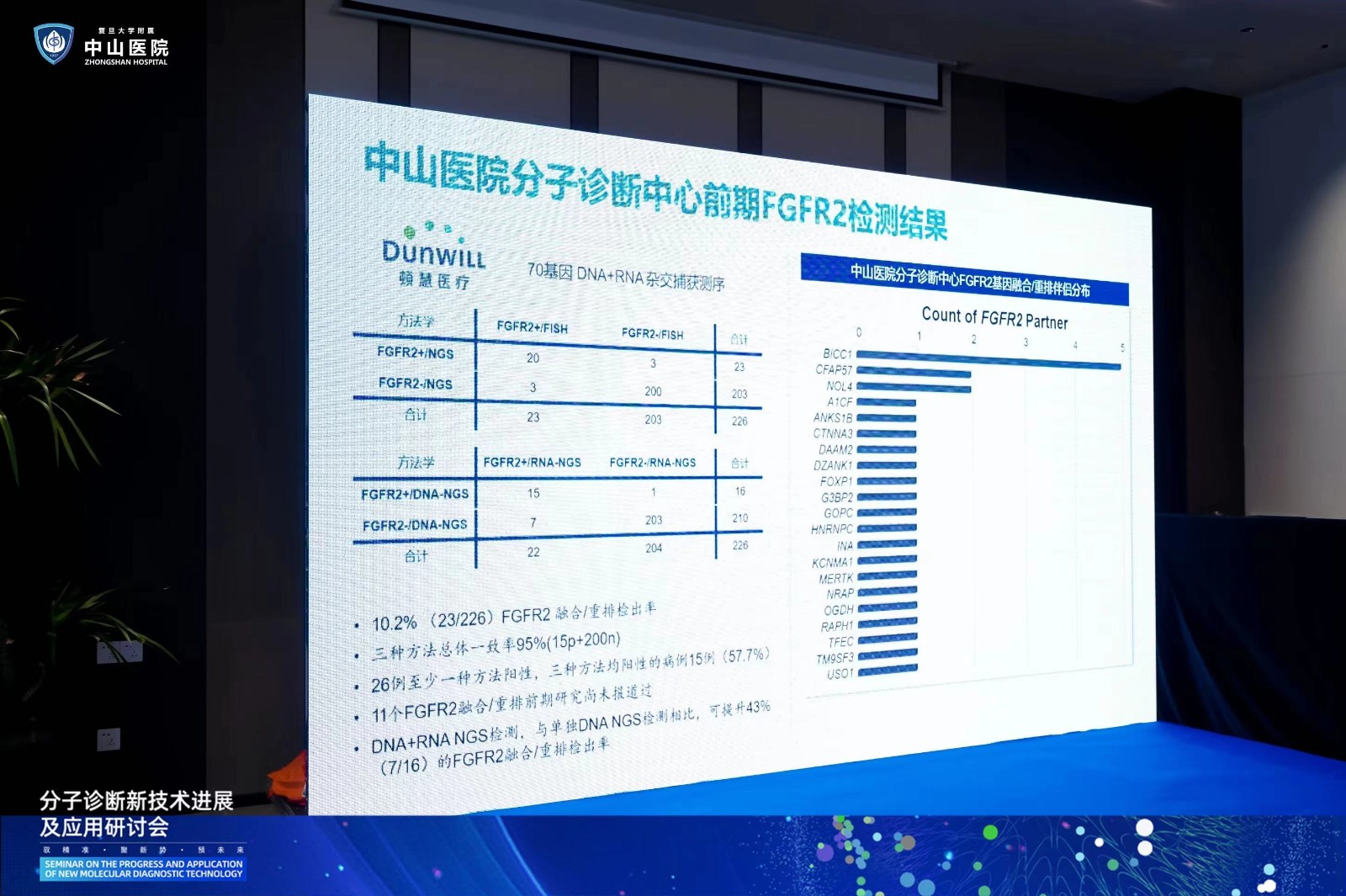
In the following discussion section, Professor Ji Yuan, Director of the Molecular Pathology Center of Zhongshan Hospital Affiliated to Fudan University, introduced the development process of a human cholangiocarcinoma gene mutation detection kit in cooperation with Dunwill Medical. The related research has been listed as one of the first HDT projects approved and filed by the National Medical Products Administration (NMPA), and has obtained a national invention patent (application number: 202311675544.3). She said: “Thanks to all the experts who participated in the multicenter research project for their participation and support, which enabled the project to quickly and officially enter the start-up stage. We also appreciate the valuable suggestions and discussions on consensus from all the experts, which enabled the project to proceed more smoothly. We believe that the FGFR2 gene fusion multicenter research project for intrahepatic cholangiocarcinoma can provide clinical pathologists with more sufficient basis for accurate detection of FGFR2 gene fusion/rearrangement in intrahepatic cholangiocarcinoma, and we hope that more and more multi methodological and multicenter studies on other target detection can be carried out in the future to jointly promote the development of precise diagnosis and treatment of intrahepatic cholangiocarcinoma.”

Finally, Professor Ji Yuan invited the attending guests to come on stage and light up the big screen of the launch ceremony together, and announcing the official launch of the "Multi center Research Project on Comparison of FGFR2 Gene Fusion/Rearrangement Detection Methods for Intrahepatic cholangiocarcinoma" project.


This conference relies on high-quality evidence-based medicine and gathers the wisdom of authoritative experts in the field of liver cancer in China. With the launch of a multicenter research project comparing the FGFR2 gene fusion/rearrangement detection methods for intrahepatic cholangiocarcinoma, it will undoubtedly propel frontline clinical doctors and pathologists to apply the "Pathological Expert Consensus on Precise Detection of Intrahepatic cholangiocarcinoma" into practice, and improve the overall diagnosis and treatment level of liver cancer in China, therefore promoting the high-quality development of the healthcare industry.





