"Jin" is Wonderful | The 2023 Branch of CCHIO Tumor Markers Special Committee Successfully Held
On November 19, 2023, the 2023 Branch of CCHIO Tumor Markers Special Committee, hosted by the Cancer Markers Committee of the China Anti-Cancer Association and organized by Tianjin Medical University Cancer Hospital, successfully concluded at the Tianjin National Convention and Exhibition Center. This meeting deeply discussed the new model of multidisciplinary integrated diagnosis and treatment from MDT to HIM, analyzed the comprehensive application of biomarkers in the integrated concept of "separation and analysis to combined treatment, from combined treatment to rational treatment", and discussed difficult and complicated cases, inspiring new thinking in multidisciplinary diagnosis of tumors.
On the afternoon of November 19th, Professor Guo Wei from the Laboratory Department of Zhongshan Hospital affiliated to Fudan University delivered the opening speech at the sub forum on "The Light of Cooperation - Transformation of Tumor Markers Products".

In the subsequent thematic presentation part, Professor Zhang Chunyan from Zhongshan Hospital affiliated to Fudan University presented a brilliant report on the “clinical application of blood miRNA in hepatocellular carcinoma”.

Director Zhang first introduced the current situation of diagnosis and treatment of liver cancer in China and the challenges it faces - China's newly developed liver cancer accounts for 42.5% of the world's cancer, its incidence rate ranks fifth in China's cancer, and its mortality rate ranks second. The overall five-year survival rate is only 11.7% -14.1%. At the time of initial diagnosis, 70-80% is already in the middle and late stages. Therefore, early detection and early treatment are the key to improving the five-year survival rate of liver cancer.

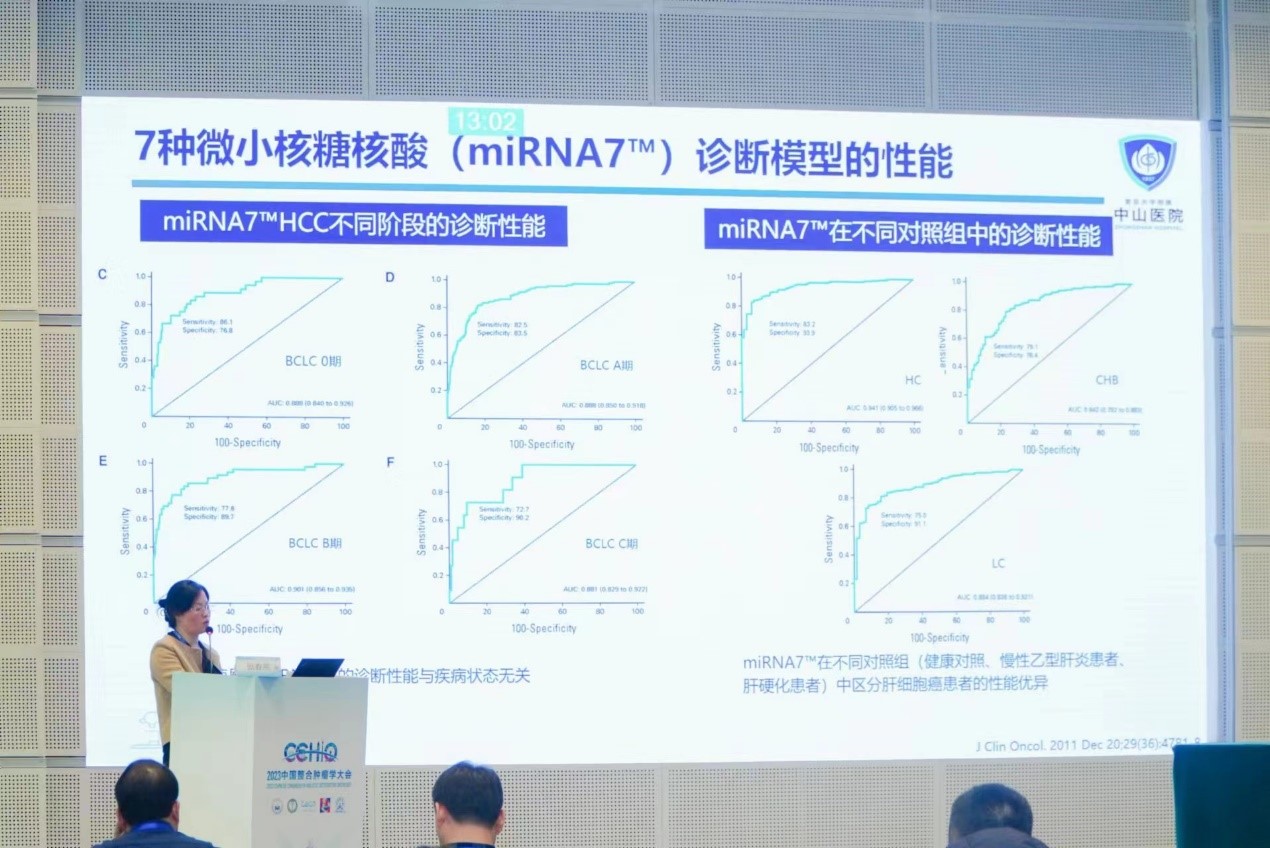
Plasma microRNAs (miRNAs), as a novel tumor marker, can stably exist in peripheral blood and have been widely studied and proven to be involved in the disease progression of liver cancer. From the perspective of clinical research value and application, Professor Zhang introduced the establishment of a liver cancer diagnostic model using seven miRNA markers in plasma, as well as the Clinical application of miRNA7™ test kits.
Early Diagnosis
MiRNA7™ demonstrated stable diagnostic performance in different clinical stages of primary liver cancer. In stage 0 Barcelona liver cancer, its sensitivity and specificity reached 86.1% and 76.8% respectively, demonstrating great potential for accurate diagnosis of very early liver cancer.
In healthy people, people with chronic hepatitis B, and people with cirrhosis, miRNA7™ is able to effectively distinguish between liver cancer patients and non-liver cancer patients, validates the solid detection performance of miRNA7™ once again.
Recurrence Monitoring
In terms of recurrence monitoring, miRNA7™ is also significantly better than other traditional liver cancer markers. Due to its minimally invasive nature, dynamic monitoring can be performed.

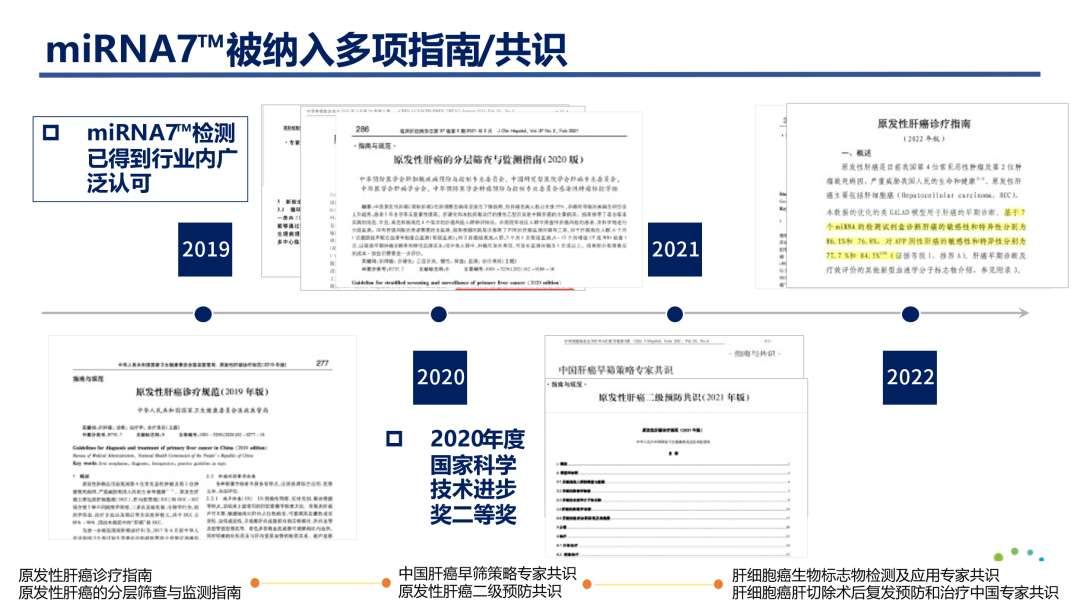
MiRNA7™ is the first liver cancer nucleic acid testing product approved by the National Medical Products Administration (NMPA), and has been included in multiple authoritative guidelines and consensus such as the Diagnosis and Treatment Guidelines for Primary Liver Cancer (2022 Edition). It has also won the second prize of the National Science and Technology Progress Award in 2020. As a clinically proven effective combination of tumor markers, and the integration with other indicators such as AFP in clinical practice, miRNA7™ will undoubtedly effectively promote the early diagnosis of liver cancer into a new era.
Finally, Director Zhang pointed out that miRNA7™ can bring multiple benefits. For patients, this project can not only detect liver cancer early, but also dynamically monitor the effectiveness of liver cancer treatment, adjust treatment plans in a timely manner, and ultimately improve the 5-year survival rate and prolong the survival time of liver cancer patients. For hospitals, this technology is at the forefront internationally and its effectiveness is superior to traditional testing methods. Conducting this project can assist in improving the diagnosis and treatment level of liver cancer in hospitals. For the society, it is conducive to the promotion of the prevention and treatment of liver cancer throughout the country, alleviating the current situation of high incidence rate and high mortality of liver cancer in China, helping to reach the goal of improving the overall five-year survival rate of cancer by 15% by 2030, which stated in the “Outline of the ‘Healthy China 2030’ Plan”. In the future, looking forward to the wider development and practice of such detection technologies in clinical practice.