The 2024 Edition of the National Health Commission's “Diagnosis and Treatment Guidelines for Primary Liver Cancer” Guangzhou Roadshow Has Successfully Concluded

On the afternoon of April 19, 2024, under the guidance of the Expert Committee for the Compilation of the Diagnosis and Treatment Guidelines for Primary Liver Cancer (2024 Edition) by the National Health Commission, and jointly organized by the China Association for Health Promotion and Education, the Liver Cancer Professional Committee of the China Anti-Cancer Association, and the China Medical Education Association, the "2024 Edition of the Diagnosis and Treatment Guidelines for Primary Liver Cancer" Roadshow was held in Guangzhou.

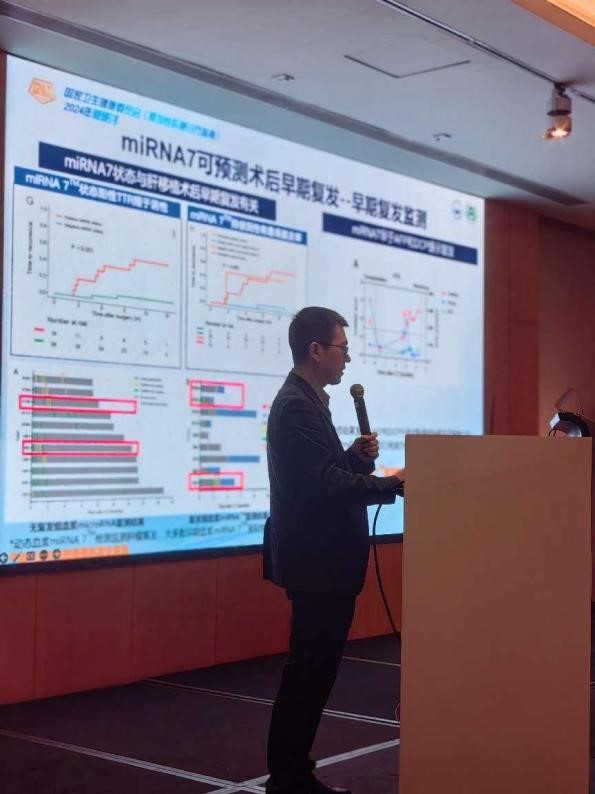
In the "Standardized Interpretation" section, Professor Dai Zhi from Zhongshan Hospital affiliated with Fudan University delivered a keynote speech on "Interpretation of Tumor Markers Diagnosis Chapter in the Diagnosis and Treatment Guidelines for Primary Liver Cancer". He first started with the global epidemiological status of liver cancer, elaborated on the current situation and challenges of liver cancer diagnosis and treatment in China, and proposed that early diagnosis and treatment of liver cancer is the key to achieving long-term survival. He further introduced the updated diagnostic pathways and technologies for liver cancer in China, as well as the new progress in tumor "liquid biopsy", which includes methods such as CTC, ctDNA, miRNA, etc.; Compared with methods such as CTC and ctDNA, miRNA has better clinical accessibility and is safer and more convenient regarding clinical use.
Then he introduced 7 microRNA test kits, miRNA7™ .The detection rate of very early liver cancer is as high as 86.1%, which is 30% higher than the traditional marker AFP. In terms of recurrence monitoring, it is also significantly better than other traditional liver cancer biomarkers, with sensitivity and specificity of 77.7% and 84.5% for AFP negative liver cancer, respectively, which is significantly better than the detection of traditional biomarkers. During and after the meeting, numerous experts communicated and discussed with Professor Dai Zhi on the clinical practice of miRNA7™.
In the part of "discussing norms with experts - deploying troops under the guidance of the new guidelines", Professor Liu Yubin from the Department of Hepatology at Guangdong Provincial People's Hospital, Professor Xie Chan from the Department of Infectious Diseases at the Third Affiliated Hospital of Sun Yat sen University, and Professor Zhang Jianwen from the Department of Hepatology at the Third Affiliated Hospital of Sun Yat sen University conducted in-depth discussions. Experts first affirmed that the emergence of liquid biopsy as a new technology can provide earlier and more accurate diagnostic services for clinical practice, because traditional detection methods such as AFP have limitations in sensitivity and specificity, and the early screening and diagnosis of miRNA7™ can play an important role in high-risk populations, and it is also a testing tool to improve the compliance of high-risk populations or postoperative patients with regular testing or follow-up visits. Meanwhile, experts also believe that there are many possibilities and more clinical value of miRNA7™ to be realized, such as miRNA7™ in HCC patients after targeted immunotherapy. The value of efficacy evaluation of miRNA7™ depend on further research and exploration by clinical experts in the future.

MiRNA7™, as the first liver cancer nucleic acid testing product approved by the National Medical Products Administration (NMPA), it has been included in multiple authoritative guidelines and consensus such as the Diagnosis and Treatment Guidelines for Primary Liver Cancer (2024 Edition). Through the content promotion and discussion of this tour, more than 100 clinical experts present and more than 10,000 online viewers have gained a better understanding of the importance of early screening, diagnosis, and treatment of liver cancer, and have also raised awareness among experts about miRNA7™ and gained a deeper understanding of how to assist clinical diagnosis and treatment. MiRNA7 ™ as a clinically proven effective combination of tumor markers, the integration with other indicators such as AFP in clinical practice will undoubtedly effectively promote the early diagnosis of liver cancer into a new era.





