Latest Release! Key Updating Points for the “Diagnosis and Treatment Guidelines for Primary Liver Cancer (2024 Edition)”
In order to further improve the standardization level of diagnosis and treatment of primary liver cancer, ensure medical quality and safety, and safeguard the health rights and interests of patients, the National Health Commission organized the revision of the "Diagnosis and Treatment Guidelines for Primary Liver Cancer (2022 Edition)", forming the "Diagnosis and Treatment Guidelines for Primary Liver Cancer (2024 Edition)", which was officially released on April 15, 2024.

The update of the screening and diagnosis section of the “Dagnosis and Treatment Guidelines for Primary Liver Cancer (2024 Edition)“ emphasizes the importance of early screening, diagnosis, and treatment of liver cancer, and proposes that liquid biopsy plays an important role in early diagnosis and efficacy evaluation of tumors. The guidelines mention a total of 12 times of the 7 microRNA combinations, which fully proves miRNA7™, the testing kit based on 7 microRNA combinations has great importance for liver cancer diagnosis.
Key Points of Update
1. Early Screening and Diagnosis
The guidelines recommend that high-risk individuals for liver cancer undergo screening at least once every 6 months. In addition to traditional screening methods such as ultrasound and AFP, the world's first liver cancer diagnostic kit, seven microRNA testing kits, miRNA7™, as an innovative screening model for liver cancer in China, has helped improve the early diagnosis rate of liver cancer and promoted early screening and diagnosis of liver cancer. For serum AFP negative individuals, DCP and 7 microRNA testing kits, miRNA7™, AFP-L3 can be used for early diagnosis.
In the 2024 version of the clinical diagnosis and roadmap for liver cancer, for high-risk individuals with liver cancer, if at least one typical liver cancer feature is found in nodules with an intrahepatic diameter of ≤ 1 cm, 1-2 cm, and>2 cm, imaging examinations can be conducted every 2-3 months for follow-up, combined with serum AFP, DCP, and 7 microRNA to confirm the diagnosis. If necessary, liver lesion biopsy can be performed.
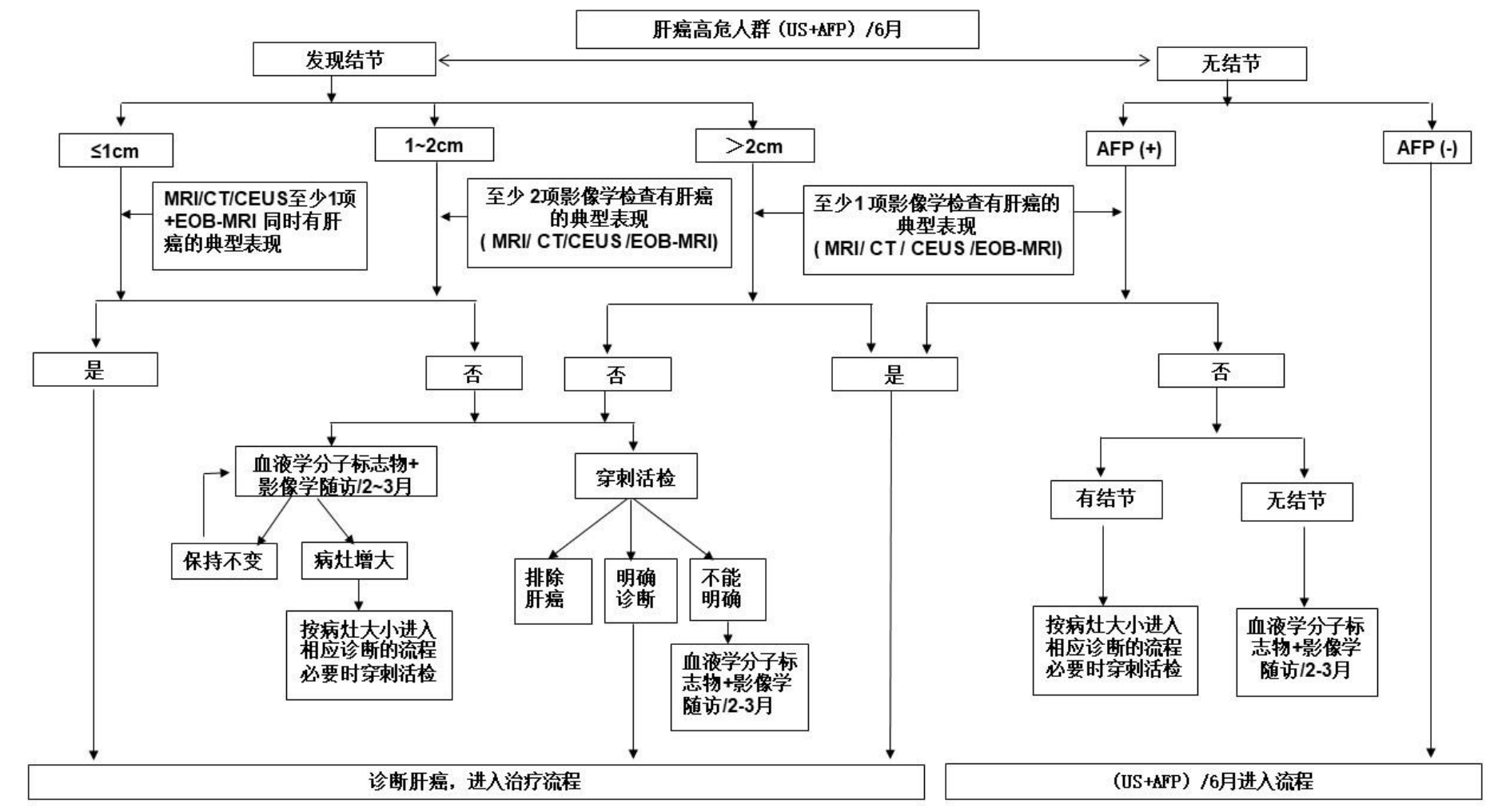
Diagnosis Roadmap for Liver Cancer
2. Sensitivity and Specificity
The guidelines indicate that the sensitivity and specificity of the testing kit based on 7 microRNA combinations for diagnosing liver cancer are 86.1% and 76.8%, respectively. The sensitivity and specificity for AFP negative liver cancer are 77.7% and 84.5%, respectively. (Evidence level 1, recommendation A).
3. Efficacy Monitoring and Recurrence Monitoring
At the same time, the guidelines have added the content of "regular follow-up for liver cancer patients after surgery" - "liver cancer patients need to have a follow-up visit 1-2 months after surgery, and then closely monitor imaging and changes in oncological markers such as AFP, DCP, and 7 miRNA combinations every 3 months. After 2 years, it can be appropriately extended to 3-6 months, and the duration is recommended for lifelong follow-up." Through such follow-up frequency and monitoring methods, the risk of recurrence in postoperative liver cancer patients can be identified as early as possible, and effective treatment methods can be adopted to improve the quality of life of patients, which also provides more powerful guideline evidence-based support for the miRNA7™ Liver cancer testing kits.
This edition of the guidelines gathers the clinical experience of numerous domestic liver cancer experts, which will further promote the overall research and clinical practice level of liver cancer in China, promote the standardization process of liver cancer in China, and promote the long-term development of standardized diagnosis and treatment of liver cancer.




