Authoritative Certification | Shanghai Clinical Laboratory Center Releases Results of External Quality Assessment for 7 microRNAs in the First Half of 2024
Recently, the Shanghai Clinical Laboratory Center (SCCL) released the results of the external quality assessment of seven microRNA projects in the first half of 2024. All Class A tertiary hospitals participating in the projects passed the evaluation 100% smoothly, confirming the stable and reliable detection performance of seven microRNA test kit, and once again shows that Dunwill's leading quality management level and precise and standardized detection technology strength have been recognized by national authoritative institutions.
The main purpose of external quality assessment is to ensure the accuracy and consistency of laboratory test results, improve medical safety, and promote mutual recognition of results between laboratories. To comprehensively test the accuracy and repeatability of miRNA7, the EQA was evaluated by 27 Class A tertiary hospitals, each providing 5 samples to detect 7 microRNAs significantly associated with liver cancer, including miR-21, miR-26a, miR-27a, miR-122, miR-192, miR-223, and miR-801, in human plasma. The detection results of miRNA7 are completely consistent with the expected results of the samples.
The quality control of in vitro diagnostic reagents in the testing process is an important guarantee for achieving accurate clinical test results. In each the testing experiment of miRNA7, quality control products and corresponding quality control standards are used to strictly evaluate the test results of quality control products according to the quality control rules, so as to ensure the authenticity and reliability of the test results of each clinical sample.
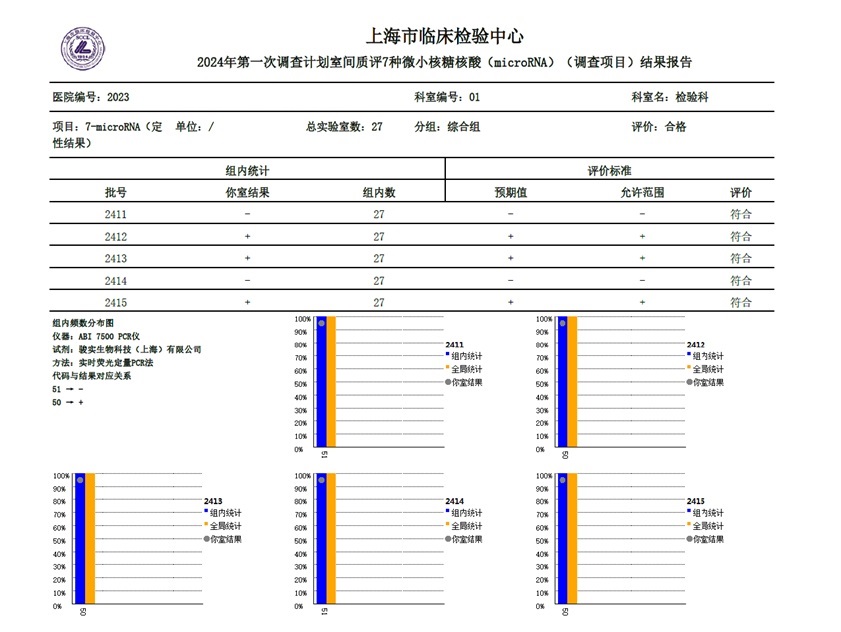
MiRNA7 has always played a unique role and value in the field of early screening and diagnosis of liver cancer due to its high sensitivity and specificity. With the increasing number of hospitals nationwide, external quality assessment has further ensured the accuracy and consistency of laboratory test results of miRNA7 among different laboratories, and ensuring the results are comparable and mutually recognized. All 27 Class A tertiary hospitals participating in this evaluation passed 100% smoothly, which also demonstrates the reliable performance of miRNA7 is not affected by different regions or laboratory environments, and it is able to continuously provide high-quality and efficient molecular detection services for clinical use.
The results of this external quality assessment are also an objective interpretation of Dunwill's rigorous quality management and excellent testing level. In the future, Dunwill will continue to uphold the service concept of “quality first”, empower clinical precision diagnosis with strict quality control standards, and continue to provide scientific, accurate, and comprehensive basis for clinical diagnosis and treatment.
About miRNA7
As the first-Class III liver cancer molecular detection product approved by the National Medical Products Administration (NMPA) in China, miRNA7 has won the second prize of National Science and Technology Progress Award in 2020 and has been included in multiple authoritative guidelines and consensus such as the "Diagnosis and Treatment Guidelines for Primary Liver Cancer (2024 Edition)".





