CTC: Beyond Counting - AI-Powered CTC Identification Model Unveils New Perspectives for Precision Cancer Diagnosis and Treatment
The recent collaboration between Dunwill Medical, Professor Yang Xinrong’s team from the Department of Hepatobiliary Surgery at Fudan University’s Zhongshan Hospital, and Professor Liu Qi’s team from the Department of Bioinformatics at Tongji University’s School of Life Science and Technology has resulted in a research paper titled “Exploring morphological heterogeneity of circulating tumor cells: machine learning-based approach for cell identification and prognostic implications” published online in the journal Science Bulletin (with an impact factor of 18.8 for 2024). This study systematically elucidates the identification of circulating tumor cells (CTCs) based on machine learning, their morphological heterogeneity, and their prognostic implications.
Abstract
Circulating tumor cells (CTCs) are the "vanguard" of tumor metastasis. They originate from the spread of primary or metastatic tumors and are crucial for understanding tumor metastasis. Compared with traditional serum biomarkers, CTCs can more directly reflect the biological characteristics of specific tumors. Therefore, they play an important role in cancer diagnosis, prognosis prediction, and treatment efficacy monitoring. The phenotypic and functional analysis of CTCs not only increases the sensitivity of cell detection but also provides key information on tumor behavior beyond mere counting. However, visual inspection of isolated fluorescently labeled CTCs is not only time-consuming and labor-intensive but also prone to errors due to reliance on human interpretation. It is also difficult to fully capture the morphological diversity of CTCs, thus ignoring their potential rich biological information. Fortunately, with the continuous progress of digital medical image analysis, artificial intelligence (AI) algorithms have provided powerful tools for in-depth analysis of CTCs. They can reveal and record a large number of morphological features, offering new opportunities for in-depth analysis of CTCs and the development of more effective treatment strategies!
CTC Recognition Model
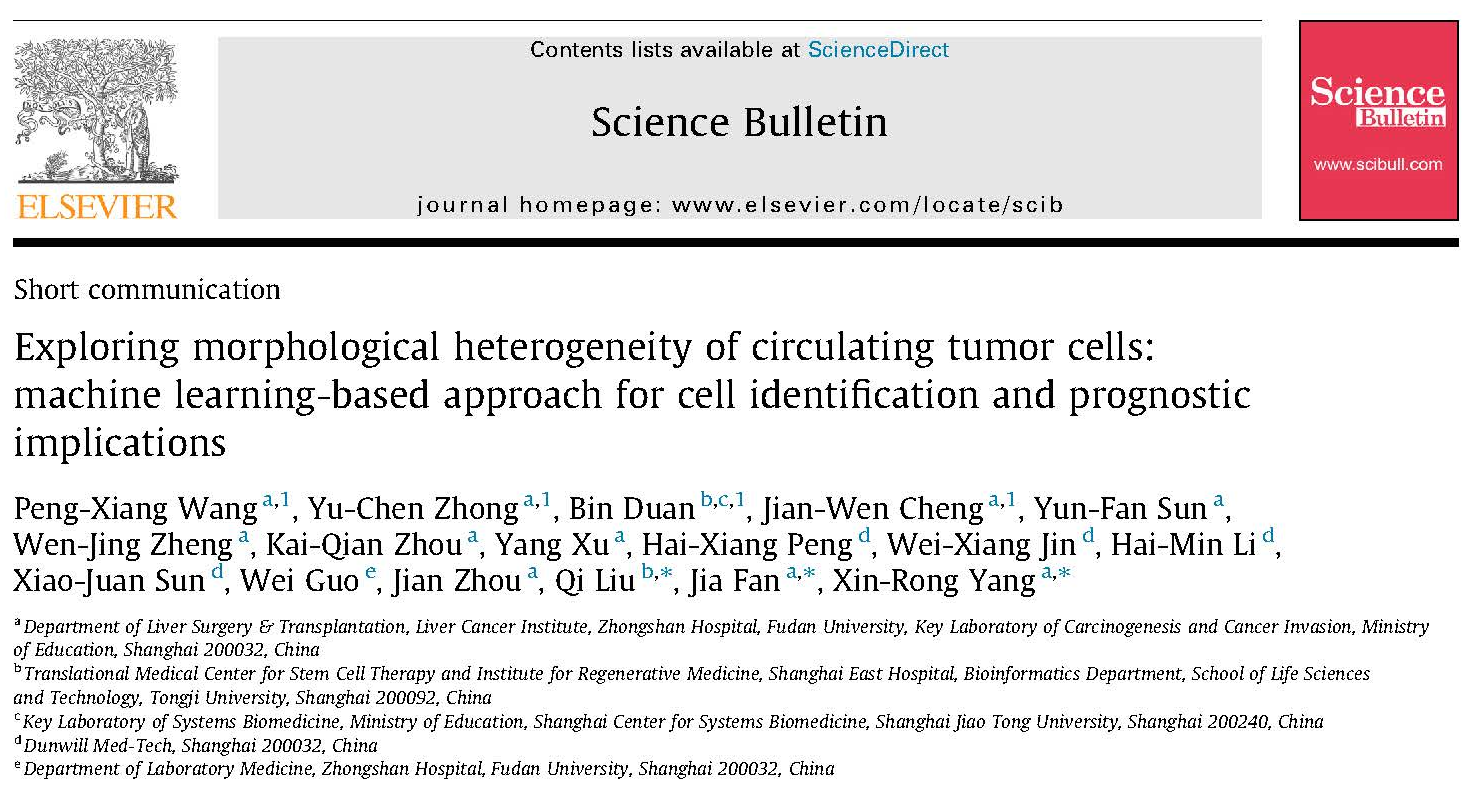
In this study, the research team utilized 9,692 CTC images and over 25 million WBC images from 1,703 patients with various cancers (including liver, gastric, biliary tract, colorectal, lung, and breast cancers). Through machine learning, they trained a CTC recognition model based on 57 extracted cell features. The model demonstrated high accuracy in CTC recognition, with an AUC-ROC exceeding 0.99 and an AUC-PR reaching 0.869. Further validation using 8,672 CTC images and over 20 million WBC images from 1,621 patients confirmed the model’s performance, with an AUC-ROC > 0.99 and an AUC-PR of 0.819. This image recognition technology is expected to achieve highly precise and efficient detection of CTCs in clinical samples.
CTC Morphological Subtyping
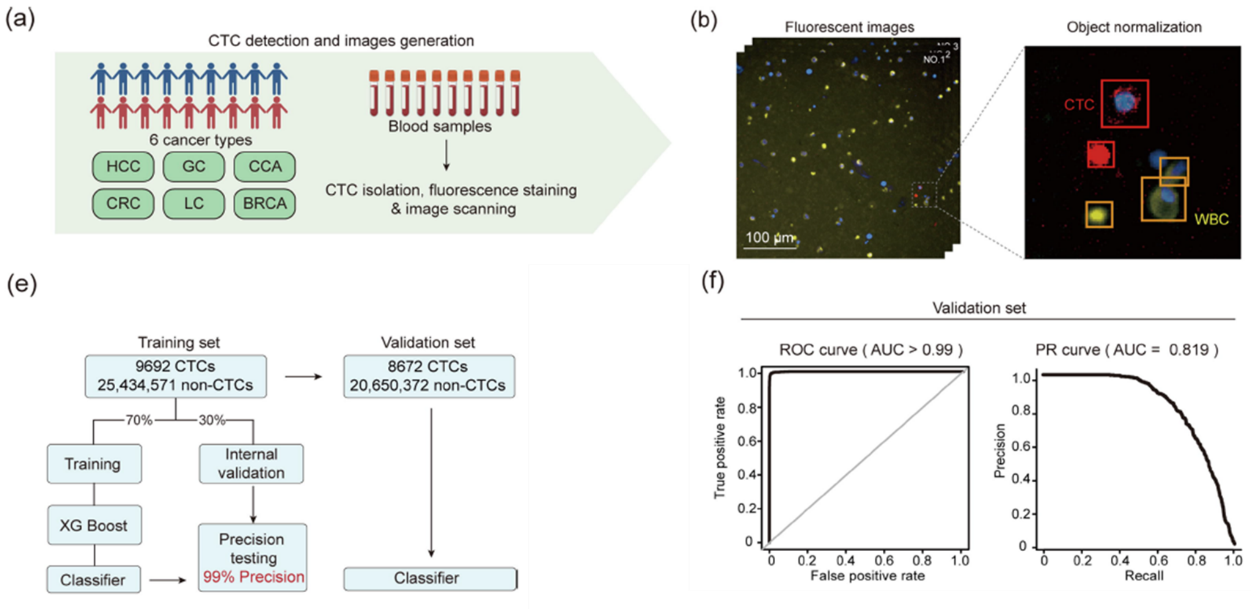
Researchers conducted an in-depth morphological analysis of CTCs from different cancer types. Contrary to the traditional view that tumor cells are usually larger than WBCs, CTCs from different cancers show significant size differences. Based on morphological features, all CTCs can be divided into six independent subgroups, each with unique characteristics: lung cancer (LC) CTCs tend to have larger nuclei and more complex cytoplasmic textures; cholangiocarcinoma (CCA) CTCs have larger diameters; breast cancer (BRCA) CTCs show stronger cytoplasmic epithelial marker fluorescence intensity; and hepatocellular carcinoma (HCC) patients' CTCs exhibit the most significant morphological heterogeneity. These differences are closely related to the histological origin of the primary tumor, providing important clues for tumor tracing.
Subtypes of CTC morphology and prognosis of HCC
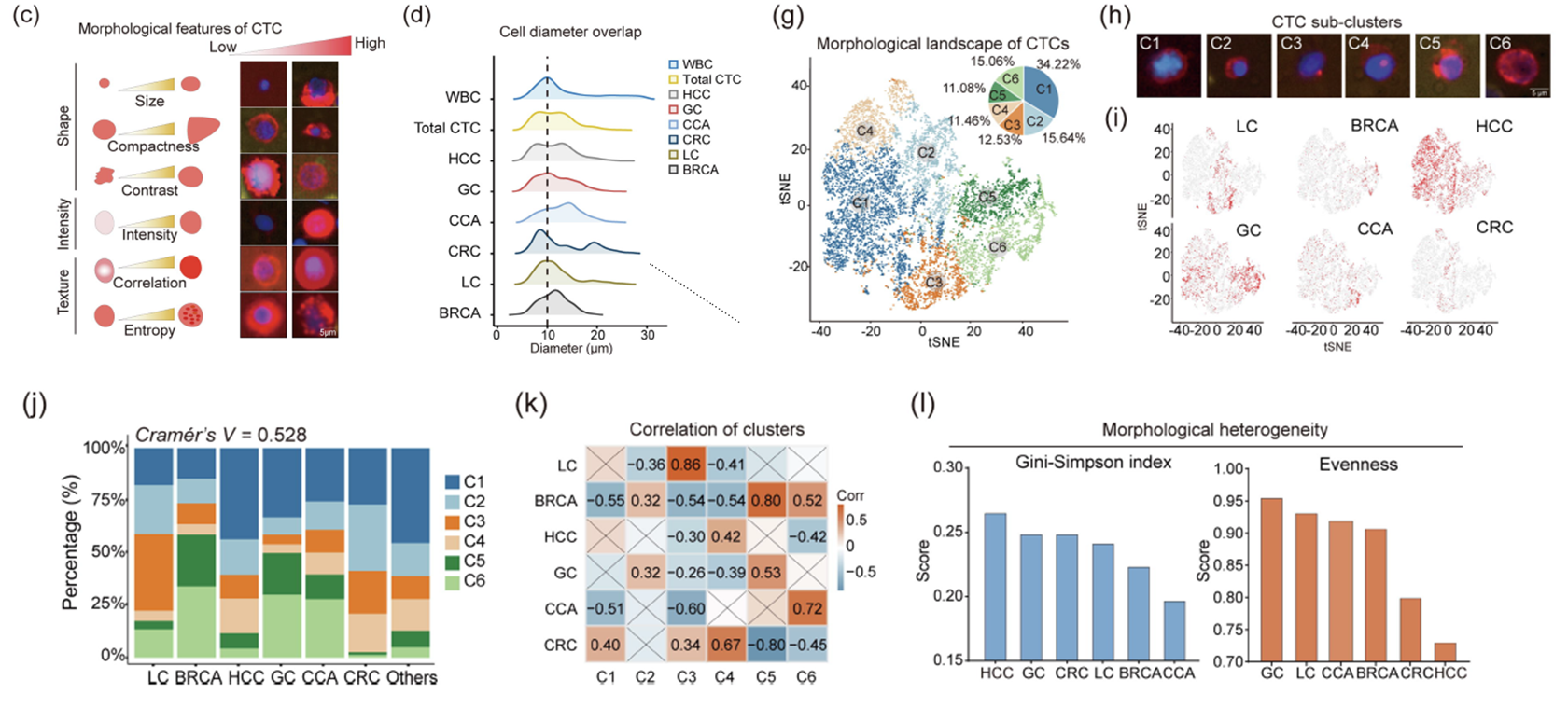
In the study of hepatocellular carcinoma (HCC), researchers identified three CTC morphological subtypes. Among them, CTCs of S-1 and S-2 subtypes are larger than those of S-3 subtype, while S-3 CTCs have higher epithelial marker fluorescence intensity and more complex texture. Patients with recurrence have significantly higher S-3 CTC burden than those without recurrence. Patients with high S-3 CTC burden have a higher risk of early recurrence within 24 months. Patients carrying only S-3 CTCs have a higher recurrence rate than those carrying only S-1 or S-2 CTCs. Patients positive for S-3 CTCs have the highest postoperative recurrence rate. In the independent validation cohort, patients with preoperative detection of S-3 CTCs have significantly shorter time to recurrence and higher recurrence rate, while S-1/S-2 CTCs have no significant impact on prognosis. Multivariate analysis shows that S-3 CTCs remain an independent prognostic factor (HR = 3.86). These findings indicate that CTC morphological subtypes can provide additional prognostic information for HCC patients. In particular, S-3 CTCs may represent a cell subpopulation with high metastatic potential and are closely related to disease progression.
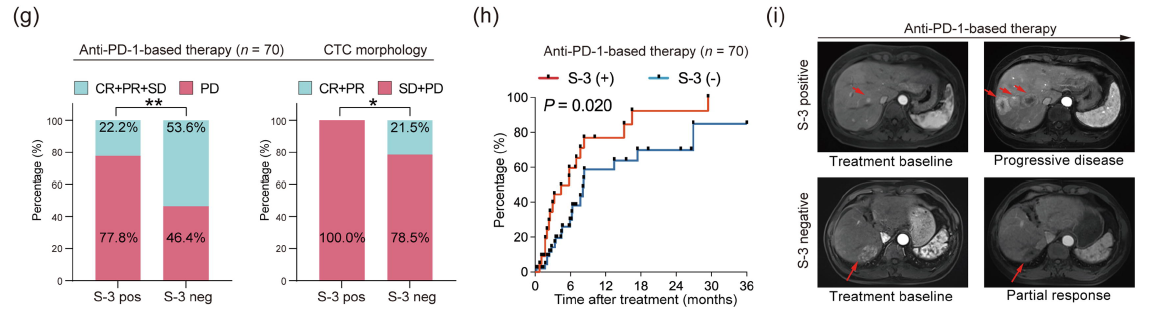
CTC Morphological Subtypes and HCC Immunotherapy Response
Researchers also explored the predictive value of CTC morphological subtypes for the immunotherapy response in HCC patients. The study found that patients with S-3 CTCs had significantly lower disease control rates and overall response rates. Moreover, patients with pre-treatment detection of S-3 CTCs had shorter progression-free intervals. Simple CTC counting could not distinguish patients' responses to immunotherapy. Tumor tissues from S-3 CTC-positive patients showed significantly reduced infiltration of CD8+ and CD4+ T cells. These results suggest that S-3 CTCs may serve as a novel predictive biomarker for immunotherapy resistance, indicating the need for enhanced monitoring and early intervention in such patients.
CTC Subtypes and Molecular Mechanisms of HCC Prognosis
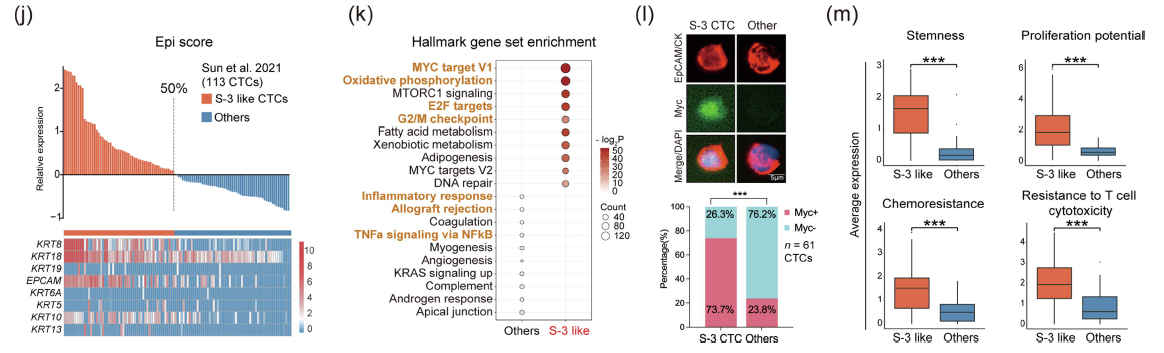
Researchers further explored the molecular mechanisms linking CTC morphological subtypes to different prognoses. They used previously published single-cell transcriptomic data of CTCs, defining the epithelial gene - expressing CTC subpopulation as high - epithelial - like S - 3 - like CTCs. These cells are significantly enriched in Myc targets, oxidative phosphorylation, E2F targets, and the G2/M checkpoint pathways. Using multi - color immunofluorescence, they confirmed Myc up - regulation in HCC patients' S - 3 CTCs. Further assessment revealed that epithelial - like CTCs have enhanced stemness, proliferation, chemotherapy resistance, and T - cell toxicity resistance, all positively correlated with epithelial characteristics. Since the retention of epithelial features is crucial for tumor cell metastasis and colonization, it is speculated that the molecular changes in S - 3 CTCs may enhance their metastatic potential.
Conclusion
The study demonstrates that the AI-based CTC recognition model significantly enhances the accuracy and feasibility of clinical CTC detection. By characterizing the unique morphological subtypes of HCC CTCs, it provides new insights into tumor biological characteristics and invasiveness, aiding in risk stratification and treatment response monitoring for HCC. This fully proves the clinical value of using CTC morphology as a novel non-invasive biomarker to advance tumor diagnosis and treatment.
In recent years, Dunwill Medical has published several important research papers in authoritative journals such as Nature Communications, Hepatology, Clinical and Translational Medicine, and Molecular Oncology. These studies have revealed cutting-edge content on CTC heterogeneity and immune escape mechanisms, providing new ideas for liquid biopsy and novel immunotherapy strategies. The ChimeraX® CTC platform of Dunwill Medical supports ultra-high-dimensional morphological research and combines AI analysis for precise CTC stratification to explore more clinical application potential. It can also be further integrated with genomics, transcriptomics, proteomics, and metabolomics for multi-omics analysis, greatly expanding the clinical and research applications of CTCs and providing more data support to uncover the mysteries of CTCs.
In the future, Dunwill Medical will continue to innovate in the field of CTC technology. By deeply collaborating with AI, it will provide more breakthrough solutions for tumor precision diagnosis and treatment, bringing health benefits to more patients.





