Dunwill Medical Makes a Brilliant Appearance in CACLP 2024, Discussed the Formulation of miRNA Test Kits Standards

The 21st China Association of Clinical Laboratory Practice Expo (CACLP) was held grandly in Chongqing recently. On the afternoon of March 16, 2024, during this annual industry event, Dunwill Medical held the "miRNA Testing Kit Standards Formulation Seminar" at the Wyndham Chongqing Yuelai Hotel, aiming to promote the development of miRNA testing technology in China, deeply interpret the new standards and regulations in the IVD testing reagent industry, and jointly explore the value and feasibility of developing industry standards for innovative biomarkers.
Ms. Sofia Wen, founder and chairman of Dunwill Medical, delivered the opening speech for the meeting: "It's a pleasure to gather with you all in the warm early spring in Chongqing. In the 18 years of steady development, Dunwill has always been committed to the discovery, development and commercialization of original biomarker, research and of original biomarkers. As the world's first launched liver cancer molecular diagnostic and testing reagent, 7 micro RNA Test Kit (miRNA7™)has been widely used in nearly 200 Class A tertiary hospitals in China and has been unanimously recognized by authoritative experts. In the face of the rapid development of the domestic miRNA detection reagent market, we have the responsibility to work together with industry leaders and experts to promote the formulation of industry standards and provide technical support for industry development and quality supervision. The purpose of this seminar is to gather ideas, explore the prospects of technological development, and promote the deeper development of miRNA detection technology in clinical applications. We are honored to invite experts and scholars from the medical laboratory industry and clinical practice to share their research results and industry experience. We also look forward to building a professional exchange and cooperation platform through this seminar, promoting the integration of "Medicine, Research, and Industry", accelerating the translation and application of miRNA detection technology, and contributing to the development of China's medical and health industry.

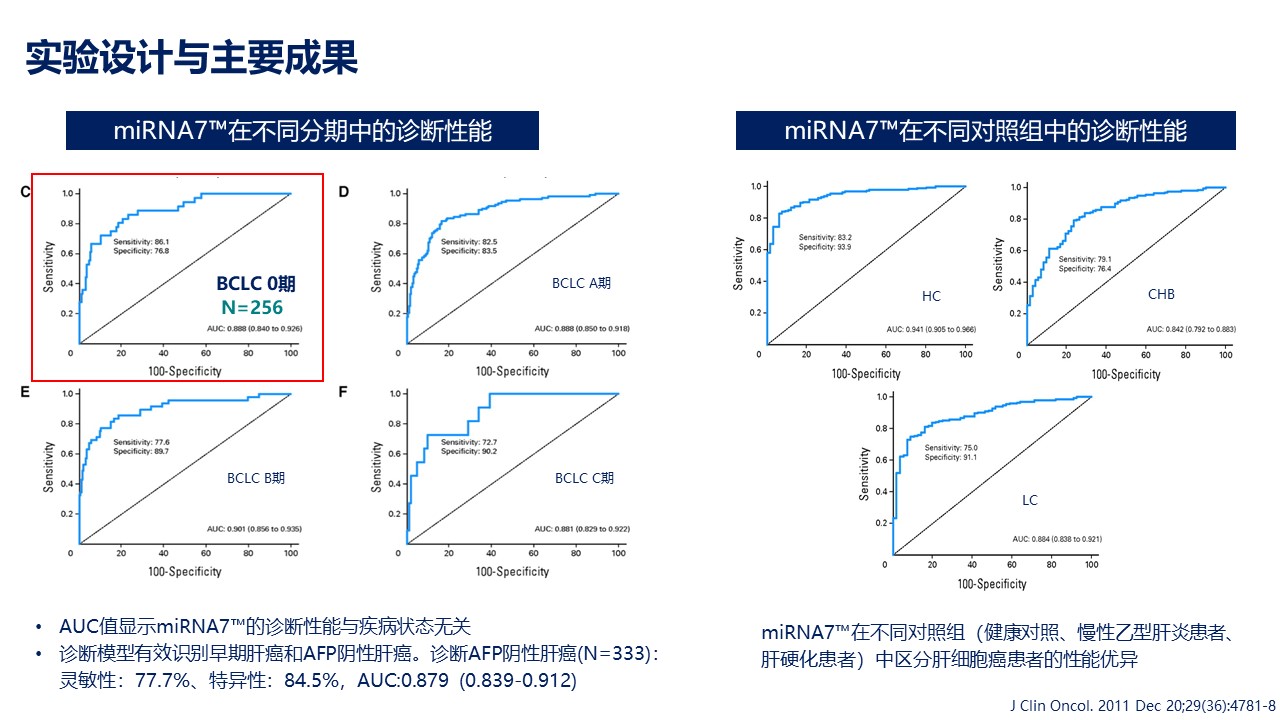
Subsequently, Professor Chunyan Zhang, Director of the Clinical Molecular Diagnosis Sub specialty at the Laboratory Department of Zhongshan Hospital Affiliated to Fudan University, presented a report titled "Application of miRNA Biomarkers in Clinical Testing". Professor Zhang believes that miRNA, as a cutting-edge tumor biomarker, has become the key to ushering in a new era of precise diagnosis and treatment of tumors. Professor Zhang introduced the establishment of a liver cancer diagnostic model using seven miRNA markers in plasma, as well as the clinical application and value of miRNA7™ Test Kit. Stable diagnostic performance of miRNA7™ is demonstrated in different clinical stages of primary liver cancer. In BCLC stage 0 liver cancer, its sensitivity and specificity reached 86.1% and 76.8% respectively, demonstrating great potential for accurate diagnosis of very early liver cancer. Among healthy people, people with chronic hepatitis B and people with cirrhosis, miRNA7™ is able to effectively distinguish between liver cancer patients and non-liver cancer patients, which once again validates the solid testing performance of miRNA7™. In terms of recurrence monitoring, miRNA7™ is also significantly better than other traditional liver cancer markers. Due to its minimally invasive nature, dynamic monitoring can be performed.

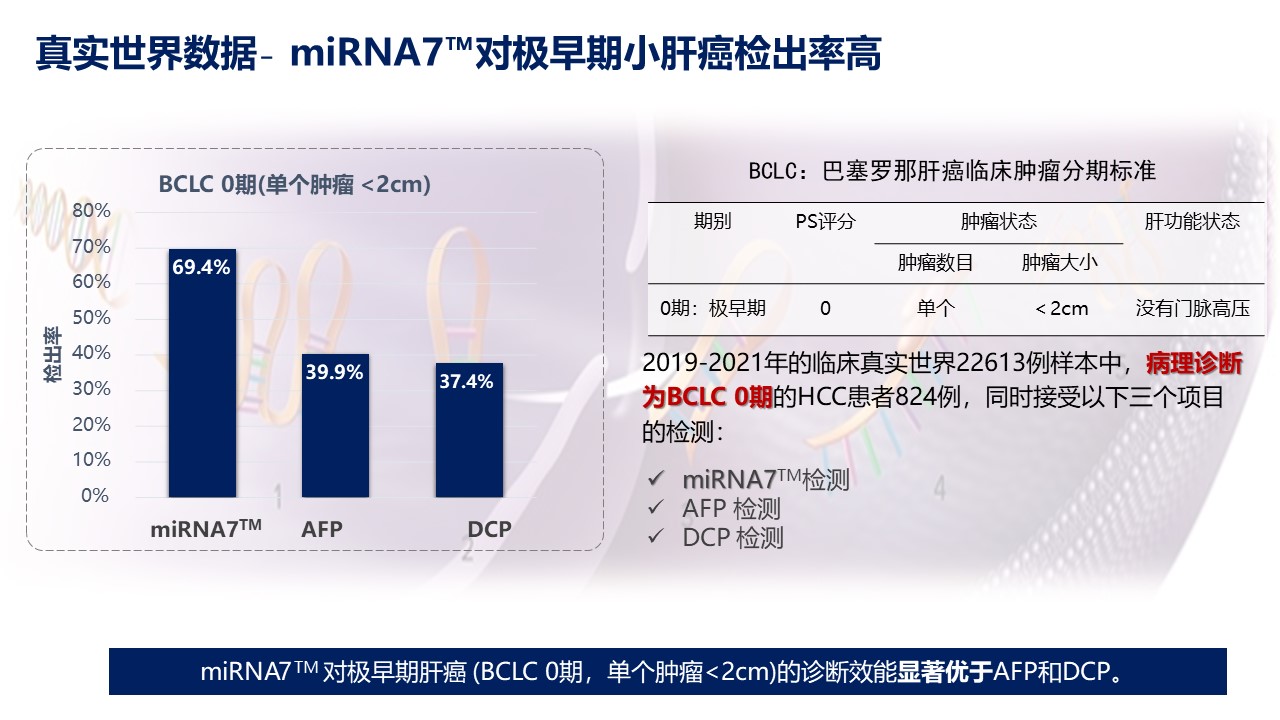
Real world research data from 2019 to 2021 shows that the detection rate of miRNA7™ of very early liver cancer (BCLC stage 0, single tumor<2cm) is 30% higher than traditional methods such as AFP.
Director Zhang stated that the hospital-Dunwill Institute for Translational Center has successfully implemented the technology translational achievement model of "Medical-R&D-Manufacturer ", and actively promoted the clinical translation process of various in vitro diagnostic products. As the first liver cancer molecular detection product approved by the National Medical Products Administration (NMPA), miRNA7™ has been included in multiple authoritative guidelines and consensus such as the Diagnosis and Treatment Guidelines for Primary Liver Cancer (2024 Edition), and is a successful example of the translation and application of innovative liver cancer biomarkers. Its widespread clinical application will undoubtedly bring benefits to more patients.

Subsequently, Xueliang Wang, Deputy Director of the Quality Control Research and Development Department of the Shanghai Clinical Laboratory Center, shared the theme report of "Multi-center Evaluation and Interpretation of 7 micro RNA test kits". Director Wang introduced that due to the influence of factors such as the testing system, personnel operation, and laboratory environment, the evaluation of detection performance of a kit by a single laboratory has certain limitations. However, adopting a multi-center parallel validation model can comprehensively and objectively evaluate the detection performance of the test kit, providing objective basis for the use and selection of reagents in clinical laboratories. Through joint evaluation and certification by 10 top tier hospitals nationwide, the precision, compliance, detection limit, and anti-interference ability of 7 miRNA test kits have shown excellent performance, which can meet the requirements and expected usages of different regions, laboratories, and detection systems, providing strong technical support for clinical diagnosis and treatment.

Yingshu Zou, Deputy Director of the In Vitro Diagnostic Laboratory of Beijing Medical Device Inspection and Research Institute, and Secretary General of the National Medical Clinical Laboratory and IVD System Standardization Technical Committee, gave a wonderful report to the audience on "From Source to End - Current Status and Development Trends of IVD Reagent Industry Standards". Director Zou gave a professional and detailed introduction on the overview of IVD standardization, quantity traceability and reference system, IVD product standardization system, medical laboratory quality and capabilities, and other aspects. Professor Zou believes that in terms of the development trend of standardization, in terms of traceability and reference systems, in the future, we will track the progress of international peers, increase our own research and development efforts, cooperate with metrology research institutions and clinical testing centers, and continue to promote the development of IVD traceability technology; In terms of IVD products, the quality of IVD reagent raw materials determines the quality of the final product. The domestic substitution of key raw materials is fully valued by the country, which will effectively promote the improvement of the quality of domestic IVD products. At the same time, interdisciplinary emerging technologies continue to emerge, and there is a strong demand for standardization. It is necessary to continue to increase the strength of standard research; In terms of medical trial quality and capability, combined with China's national conditions and practical experience, the universality requirements of ISO15189 will be concretized in various disciplines, and the standardized implementation of laboratory testing will be explored.

During the roundtable discussion, hosted by Zhanjie Liu, the General Manager of JUSBIO SCIENCES (SHANGHAI) CO.Ltd., the guests had a lively discussion on the topic of "the value/feasibility of developing industry standards for innovative biomarkers". They shared their professional insights and valuable experiences, and made many forward-looking and targeted suggestions.

Finally, Professor Hualiang Wang, Dean of Shanghai Experimental Medicine Research Institute, delivered a concluding speech for the conference. He expressed his gratitude to Ms. Sofia Wen, founder of Dunwill Medical, and her team for carefully preparing this high-level academic seminar. He also thanked all experts and attending representatives for their support and participation. miRNA, as an ideal biomarker for blood testing, has broad application prospects in disease diagnosis, prognosis evaluation, and treatment monitoring. With the development of technology, the research and application of miRNA test kits are becoming more and more extensive. Especially the miRNA7™ of Dunwill Medical, the liver cancer test kit has been applied in nearly 200 Class A tertiary hospitals nationwide, bringing hope for early diagnosis and treatment of high-risk groups for liver cancer, and setting a benchmark for other enterprises in the industry. The professional insights and valuable opinions of today's experts are crucial for the future development of scientific and reasonable industry standards for miRNA test kits. He believes that with everyone's joint efforts, we will accelerate the innovative research and rapid development of miRNA related biomarkers in China, and make greater contributions to the people's health cause.

The experts at this meeting had in-depth discussions and exchanges on the importance and enormous potential of miRNA detection technology in the medical and health field, the advantages and progress of clinical applications, as well as the challenges of industry standard formulation and quality supervision, which brought us a series of positive results and laid the foundation for the development of China's miRNA detection reagent industry.
The sacred mission of Dunwill is to drive medical development through technological innovation and powering the future of medicine. Taking exploration as its wings and moving towards the future, Dunwill will persistently expand the depth and breadth of technological innovation, assist in the significant development of miRNA detection technology in the field of medical and health, and jointly look forward to, think about, and promote the infinite possibilities of improving national health.





