Advancing Together Under the Guidance of Guidelines | The 7th Yangtze River Delta Liver Disease Conference and Multidisciplinary Diagnosis and Treatment Academic Seminar on Liver Tumors were Successfully Held
Sponsored by Beijing Chen Jumei Public Welfare Foundation, Beijing Health Promotion Association, Zhongguancun Zhuoyi Chronic Disease Prevention and Technology Innovation Research Institute and Shanghai Oriental Infectious Disease Prevention and Rehabilitation Research Center, and organized by Shanghai infectious diseases Clinical Quality Control Center, Shanghai Jiaotong University Medical College Ruijin Hospital Multidisciplinary Joint Diagnosis and Treatment Center for Liver Cancer, and Shanghai Jiaotong University Medical College Critical Viral Hepatitis Clinical Diagnosis and Treatment Center, the Seventh Yangtze River Delta Liver Disease Conference and Multidisciplinary Diagnosis and Treatment Academic Seminar for Liver Cancer were successfully held in Shanghai recently. The conference gathered renowned experts from infectious diseases, hepatology, imaging, hepatobiliary surgery, and oncology from the Yangtze River Delta region and across the country to give thematic reports and share case studies on cutting-edge clinical information and treatment guidelines in various disciplines, and jointly discuss multidisciplinary joint diagnosis and treatment strategies for liver diseases and liver tumors.

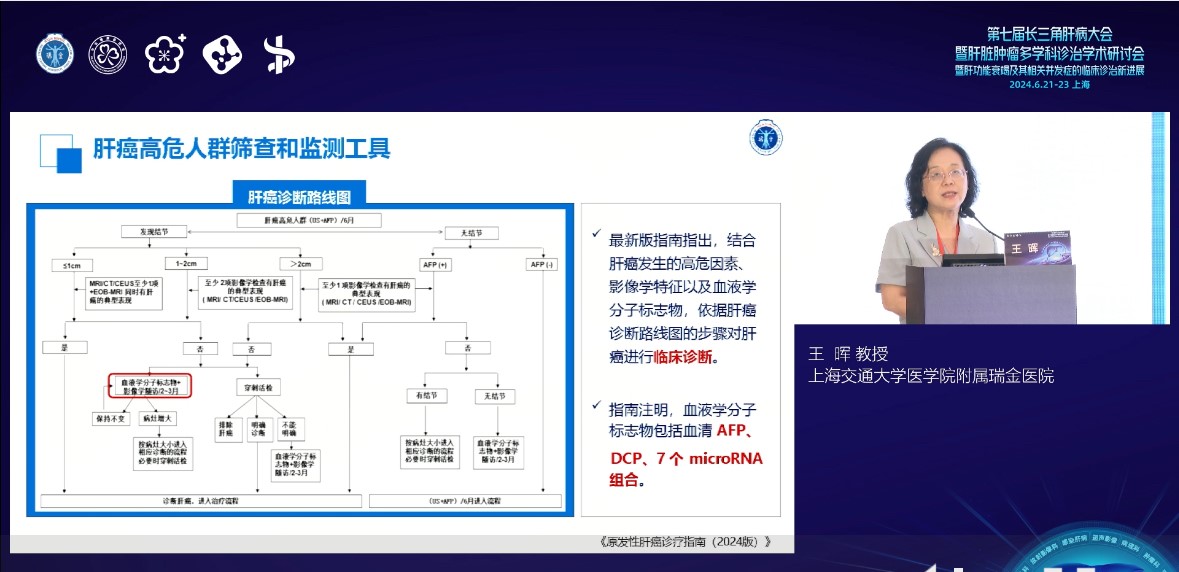
On the afternoon of June 22nd, Professor Wang Hui, Director of the Department of Infectious Diseases at Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, delivered a keynote speech titled "Stratified Screening of "Liver Cancer - from Guidelines to Clinical Practice" at the "Early Screening and Early Diagnosis, Innovative Progress" segment of the multidisciplinary standardized development branch venue of liver disease at the conference, which received widespread praise from the attendees.
Director Wang pointed out that domestic and foreign studies have shown that regular screening for liver cancer can help with early diagnosis and treatment, bringing survival benefits to patients. According to the Diagnosis and Treatment Guidelines for Primary Liver Cancer (2024 Edition), high-risk populations for liver cancer need to be stratified according to the risk of liver cancer occurrence. For high-risk populations for liver cancer, it is recommended to undergo routine screening at least once every 3-6 months. The new version of China's liver cancer diagnostic roadmap clearly identifies 7 microRNA combinations as early diagnostic biomarkers for liver cancer, and points out that clinical diagnosis of liver cancer should be carried out based on the steps of the liver cancer diagnostic roadmap, taking into account high-risk factors for liver cancer occurrence, imaging features, and hematological molecular markers. In addition to traditional screening methods such as ultrasound and serum alpha fetoprotein (AFP), China's first liver cancer molecular detection product approved by the National Food and Drug Administration includes seven microribonucleic acid detection kits, As an innovative screening model for liver cancer in China, miRNA7™ can significantly improve the detection rate of very early liver cancer and promote early diagnosis and treatment of liver cancer.

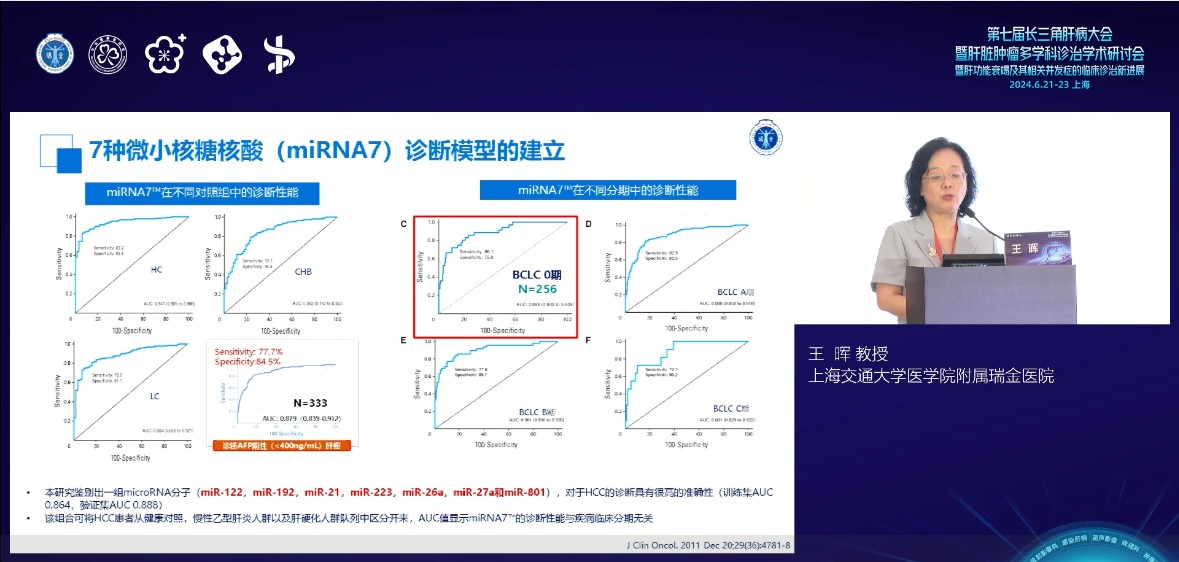
Subsequently, Director Wang introduced the establishment of a liver cancer diagnostic model using seven plasma miRNA markers from the perspectives of clinical research value and application, as well as the clinical application of miRNA7™ testing kits. After screening and validation through thousands of large-scale studies, it is proved that the diagnostic model of miRNA7™ can effectively identify very early liver cancer and AFP negative liver cancer. The detection rate of miRNA7™ for very early liver cancer is as high as 86.1%, which is 30% higher than the traditional marker AFP; The sensitivity and specificity for AFP negative liver cancer were 77.7% and 84.5%, respectively, which were significantly better than the detection of traditional biomarkers. MiRNA7™ can effectively identify people with primary liver cancer from healthy people, people with hepatitis B and people with liver cirrhosis, and shows stable performance.
The data of miRNA7™ in the clinical real world is also excellent. According to the clinical real world sample data of 22613 cases from 2019 to 2021, the diagnostic efficacy of miRNA7™ in very early liver cancer is significantly better than AFP and DCP. Therefore, miRNA7™ can greatly reduce the missed diagnosis of early liver cancer patients due to AFP negative in clinical practice, improve the overall detection rate of very early liver cancer, provide more accurate diagnosis for clinical doctors, and bring valuable treatment time to patients.

Finally, Director Wang stated that miRNA7™ has significant advantages in the full process management of liver cancer. In terms of clinical application, miRNA7™ can significantly improve the detection rate of very early liver cancer and is also significantly superior to other traditional liver cancer biomarkers in recurrence monitoring, especially for AFP negative populations. In addition, the 2024 version of the "Guidelines" mentions a total of 12 microRNA combinations, which fully proves the importance of the testing kit miRNA7™,based on 7 microRNA combinations to liver cancer diagnosis.

The influence of this conference covers the Yangtze River Delta and even multiple provinces and cities across the country, effectively promoting the level of multidisciplinary joint diagnosis and treatment as well as the integration of high-quality diagnosis and treatment of liver diseases and liver tumors in the Yangtze River Delta region, promoting the full course management of liver tumors and the implementation of multidisciplinary diagnosis and treatment "gold" standards, and strengthening the collaborative cooperation and common progress of the Yangtze River Delta Alliance.
Through the communication and sharing at this meeting, nearly 200 clinical experts and deans present, as well as over 7000 online clinical doctors, have gained a deeper understanding of the updated key points of the new guidelines and the application and value of miRNA7™ in the precision diagnosis and treatment in clinical practice. We believe that with the promotion and implementation of standardized processes for liver cancer diagnosis and treatment in the future, it will promote the homogenization of medical levels in various regions of China, improve the treatment effectiveness and quality of life of more patients, and help China's liver disease industry achieve high-quality and high-level development.





